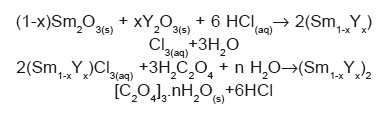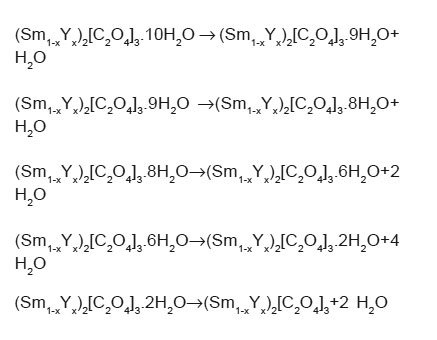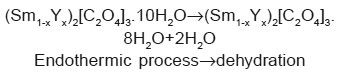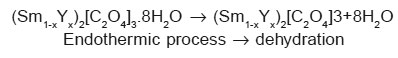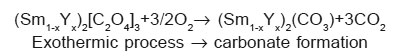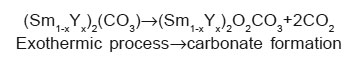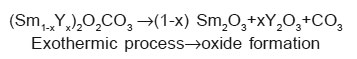Syntheses and Thermal Characterisation of Mixed (Sm-Y) Oxalates Prepared by Coprecipitation Method
Presenjit Pauri1, S. K. Ghoshal2 and H. S. Tewari1*
1Department of Pure and Applied Physics, Guru Ghasidas Vishwavidyalaya, Koni, Bilaspur - 495 009, India.
2Department of Physics, Faculty of Science, Universiti Teknologi Malaysia - 81310 UTM Skudai, Johor.
DOI : http://dx.doi.org/10.13005/msri/080116
Article Publishing History
Article Received on : 17 Mar 2011
Article Accepted on : 20 Apr 2011
Article Published :
Plagiarism Check: No
Article Metrics
ABSTRACT:
In this work, mixed (Sm1-xYx)2[C2O4]3.nH2O with x= 0.50 and 0.70 were prepared using standardized co precipitation method. All the synthesized oxalates were characterized by x-ray diffraction and differential scanning calorimeter. All the samples are homogeneous in stoichiometry. The XRD analysis of these synthesized samples reveals that a solid solution forms for these two compositions The thermal decomposition of the mixed oxalates is complex although completed a lower temperatures. This is an important result for the preparation of Sm-Y mixed oxides, because all the properties of the mixed oxides are intrinsic and depend only on their composition and thermal treatment schedules.
KEYWORDS:
Thermal characterisation; Oxalates; Coprecipitation method
Copy the following to cite this article:
Pauri P, Ghoshal S. K, Tewari H. S. Syntheses and Thermal Characterisation of Mixed (Sm-Y) Oxalates Prepared by Coprecipitation Method. Mat.Sci.Res.India;8(1)
|
Copy the following to cite this URL:
Pauri P, Ghoshal S. K, Tewari H. S. Syntheses and Thermal Characterisation of Mixed (Sm-Y) Oxalates Prepared by Coprecipitation Method. Mat.Sci.Res.India;8(1). Available from: http://www.materialsciencejournal.org/?p=2511
|
Introduction
Mixed rare earth oxides and oxalates have gained importance due to their various properties mainly related to their ionic conductivity, magnetic or dielectric properties.1,2,3 Rare earth oxides are interesting materials in technical fields such as catalysts for a large number of organic reactions, host materials for powerful lasers or as precursors for magnetic and superconducting materials.4,5 Generally, it is difficult to synthesis homogeneous mixed rare earth oxide following a popular high temperature conventional solid state ceramic method because these oxides are highly thermal stable and low diffusion coefficients of cations. An important route for synthesis of these mixed oxides is the thermal decomposition of previously synthesized organic or inorganic salts like acetates, oxalates or carbonates.6-9 In these salts when cations are homogeneously mixed, the composition leads to a mixed oxide. These compounds decompose at relatively low temperature into a mixed oxide. The kinetics of dehydration and decomposition is often very complex and undergoes many intermediate steps before final decomposition into mixed oxides.
A very little literature is available on the thermal decomposition of mixed oxalate systems.3, 11-13 In the present work we are reporting the synthesis and thermal decomposition of interesting mixed oxalates of Samarium and yttrium. Both Sm2O3 and Y2O3 are cubic at room temperature. In this work, mixed (Sm1-xYx)2[C2O4]3.nH2O with x= 0.50 and 0.70 were prepared using standardized coprecipitation method. All the synthesized oxalates were characterized by x-ray diffraction and differential scanning calorimeter.
Experimental
All the samples were prepared by a co-precipitation method, starting from Sm-2O3 (99.9% pure, LOBA Chemicals) and Y2O3 (99.9% pure, LOBA Chemicals) powders. They were weighted in order to have a value of x from 0.50 to 0.70. A solution was prepared from these oxides using a slight excess of HCl. In order to ensure a complete precipitation and to avoid any possible concentration gradient, the precipitation of the mixed oxalate was achieved by fast addition of a fresh prepared oxalic acid solution in large excess. The precipitate was filtered, washed with distilled water till no trace of the Chloride ion was detected and then dried in air at an 80OC for 24 hours. The structure of all these samples was investigated by x-ray powder diffraction using a Rigaku MiniFlex diffractometer (Cu Kα radiation) in the range 20≤2≤60 at room temperature. A Shimadzu DSC – 60 Differential Scanning Calorimeter was utilized to study the kinetics of dehydration and decomposition of these synthesized oxalates up to 550 ºC in air.
Results and Discussion
Powder Synthesis and Characterization
According to our experimental procedure rare earth oxalate form following the reaction path:
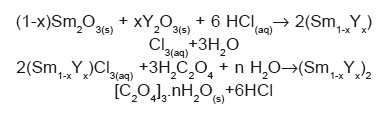
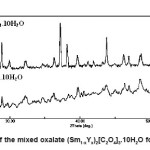
Mixed oxalates start to precipitate immediately after addition of oxalic acid in mixed chloride solution rare earth oxides because these oxalates are highly insoluble in water. The shape, size and distribution of precipitated particles are composition dependent. When the freshly prepared oxalic acid solution was added to mixed chloride solution of rare earth oxides, even if it is vigorously stirred, the conditions for formation of rare earth oxalates instantaneously achieved, as the concentration of oxalate ions is locally high. Due to this large number of nuclei formed in a small region and grow quickly forming an aggregates. The nucleation and growth rate mechanism is different for these two oxalates and is dependent on the ratio of Sm and Y ions in solution. For these compositions, variation of shape and the size of the aggregates depend on ratio of Sm/Y cations in the samples. X-ray diffraction patterns of these samples confirm these observations as shown in Fig.1. The precipitated mixed oxalate for the composition x=0.50 is more crystalline in nature in comparison to the composition corresponding to x=0.70 in the system (Sm1-xYx)2[C2O4]3.nH2O. From these diffraction patterns, we also conclude that a complete solid solution between Sm2O3 and Y2O3 forms for compositions corresponding to x=0.50 and 0.70. The properties of these oxalates also depend on composition only. A similar behaviour has been reported also for other oxalates.3,10,14,16
Dehydration and Decomposition Analysis
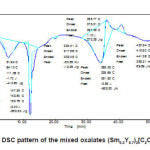
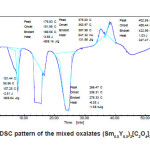
The kinetics of dehydration and thermal decomposition of these mixed oxalates in to their oxides is complex and undergoes many intermediate steps. Figs. 2-3 show the differential scanning calometry (DSC) analysis. In these patters, a number of endothermic and exothermic peaks are observed indicating that many reactions occur during decomposition of these oxalates.
According to thermal analysis, rare earth oxalates easily lose water at low temperature and CO2 at higher temperature below 7000C. In general, decomposition procedure accomplished through three important thermal stages: loss of adsorbed water; water of dehydration and decomposition of anhydrous oxalates into respective oxides.3,14 It has been reported in literature that the hydration water can be fully recovered when kept in a moist environment.3,10 In the system (Sm1-xYx)2 [C2O4]3.nH2O with x=0.3 and x=0.5 ,the DSC patterns show number of endothermic reactions at low temperatures and two exothermic reactions at higher temperature regions. The endothermic reaction at lower temperatures show the loss of water of hydration associated with rare earth oxalates.
On the strength of the data, we can state that the dehydration path of (Sm1-xYx)2[C2O4]3.nH2O is consistent with the following3,10,14:
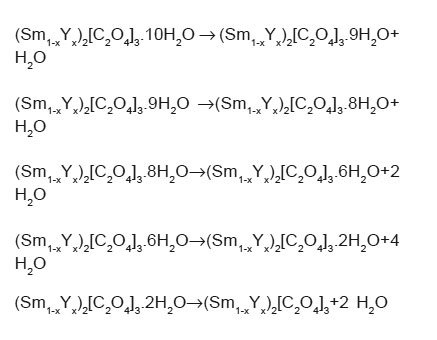
The endothermic reactions at higher temperature may be due to decomposition of oxalate in oxides. So it is possible to propose the decomposition of (Sm1-xYx)2[C2O4]3.10H2O as follows:
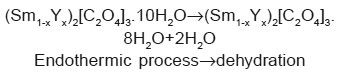
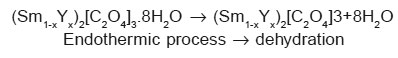
The exothermic reaction in high temperature region may be due to phase formation of the mixed rare earth oxides. Anhydrous oxalates decomposed first in (Sm1-xYx)2[C2O4]3 then (Sm1-xYx)2O2CO3 and finally in the mixed oxides for which the temperature is beyond the limit of the instrumentation used.
The exothermic reaction can be written as follows3, 18:
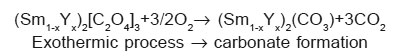
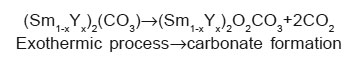
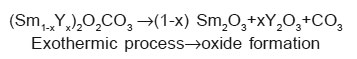
The variation in onset and end set of various reaction of two DSC patterns is due to change in composition of rare earth oxalates formed. The thermal behaviour of oxalates is similar even if the temperatures of all the transformations increase with yttrium concentration. The thermal stability of the products depends on the molecular mass of the oxalates. The positions and the forms of the peaks which is composition dependent confirm that the chemical bond with water is different for these two compositions.19, 20
Figure 1: XRD pattern of the mixed oxalate (Sm1-xYx)2[C2O4]3.10H2O for x = 0.50 and x = 0.70
Figure 2: DSC pattern of the mixed oxalates (Sm0.3Y0.7)2[C2O4]3.10H2O
Figure 3: DSC pattern of the mixed oxalates (Sm0.5Y0.5)2[C2O4]3.10H2O
Conclusions
The two samples of Sm-Y mixed oxalates were synthesized by using co precipitation method. The XRD and Differential Scanning Calorimetric analysis were performed to study the phase formation and thermal decomposition behaviour of these two compositions. The XRD analysis of these synthesized samples reveals that a solid solution forms for these two compositions and Sm rich sample is more crystalline in the system (Sm1-xYx)2[C2O4]3.10H2O with x = 0.50 and x = 0.70. The thermal decomposition of the mixed oxalates is complex although completed a lower temperatures. The decomposition process of these oxalates goes through three important stages. These oxalates first decompose in (Sm1-xYx)2(CO3), then in (Sm1-xYx)2O2CO3 and finally in the mixed oxides. This is an important result for the preparation of Sm-Y mixed oxides, because all the properties of the mixed oxides are intrinsic and depend only on their composition and thermal treatment schedules.
Acknowledgements
The authors are very grateful to department of Pure and Applied Physics, Guru Ghasidas Vishwavidyalaya, Koni, Bilaspur (C.G.), India for necessary experimental facilities used in the present work. One of us (HST) is also thankful to Dr K D Mandal, Department of Applied Chemistry, Banaras Hindu University, Varanasi for his helpful contributions.
References
- Hussein. G. A. M., J. Anal. Pyrol. Sci., 37:111 (1996).
- Elizabeth A., Joseph C., Paul I., Ittyachen M. A., Mathew K. T., Lonappan A. and Jacob J., Mater. Sci. Eng. A, 391: 43 (2005).
CrossRef
- Ubaldini A., Artini C., Costa G. A., Carnasciali M. M. and Masini R., J. Therm. Anal. Cal., 91: 297 (2008).
- R. Reisfeld and K. Jorgensen, Lasers and Excited States of Rare Earths, Springer, New York (1978).
- P.Mele, C. Artini, R. Masini, G. A. Costa, A. Hu, N. Chikumoto and M. Murakami, Physica C, 391: 49 (2003).
CrossRef
- R. D. Purohit, S. Saha and A. K. Tuagi, Ceram. Int., 32: 143 (2006).
CrossRef
- M. Popa and M. Kakihana, Solid State Ionics,141-142: 265 (2001).
CrossRef
- C. Peng, Y. Zhang, Z. W. Cheng, X. Cheng and J. Meng, J. Mater. Sci. Mater. Electr., 13: 757 (2002).
CrossRef
- J.-G. Li, T. Ikegami, Y. Wang and T. Mori, J. Solid State Chem., 168: 52 (2002).
CrossRef
- A. Ubaldini, C. Artini, G. A. Costa, M. M.Carnasciali and R. Masini, J. Therm. Anal.Cal., 84: 207 (2006).
CrossRef
- M. Popa and M. Kakihana, Solid State Ionics,141-142: 265 (2001).
CrossRef
- G. A. M. Hussein, M. H. Khedr and A. A.Farghali, Colloids Surf. A, 203: 137 (2002).
CrossRef
- D. Zhan, C.Cong, K. Diakite, Y. Tao and K. Zhang, Thermochim. Acta, 430: 209 (2003).
- Gallagher S. A. and Dworzak W. R., J. Amer. Ceram. Soc., 68: C-206 (1985).
CrossRef
- Vaidya S., Ahmad T., Agrawal S. and Ganguli A. K., J. Amer. Ceram. Soc., 90: 863 (2007).
CrossRef
- Coetzee A., Brown M. E., Eve D. J. and Strydom, J. Thermal Analysis, 41: 357(1996).
CrossRef
- B. A. A. Balboul, Thermochim. Acta, 351: 55 (2000).
CrossRef
- K. G. Nair, V. V. Sreerajan and V. S. V. Nayar,Thermochim. Acta, 39: 253 (1980).
CrossRef
- G. A. Tompsett, R. J. Phillips, N. N. Sammesand A. M. Cartner, Solid State Commun.,108: 655 (1998).
CrossRef
- J. Tong and L. Eyring, J. Alloys Compd., 225:139 (1995).
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal