Sridhar Pande1, Arunkumar Lagashetty2, C. D. Madhusoodana3 and A. Venkataramana1*
1Department of Materials Science, Gulbarga University, Gulbarga, Karnataka, India.
2Department of Chemistry, Appa Institute of Engineering and Technology, Gulbarga, Karnataka, India.
3C.B.U, Bharat Heavy Electricals Ltd. Bangalore, Karnataka, India.
DOI : http://dx.doi.org/10.13005/msri/080212
Article Publishing History
Article Received on : 10 Jun 2011
Article Accepted on : 20 Jul 2011
Article Published :
Plagiarism Check: No
Article Metrics
ABSTRACT:
Chemistry of earth materials directs its applications, which enhances the science and technology of materials. Bentonite clay mineral belongs to the smectite group having wide range of chemical composition. Crystal size and chemical composition of clay is responsible for several properties and applications. In the present study, clay sample is collected from Gulbarga mine and is made active by acid treatment with sulphuric acid, nitric acid and phosphoric acid. The structure of the Bentonite clay before and after acid treatment is studied by X-ray diffraction study (XRD), morphology of the sulphuric acid treated clay is studied by Scanning Electron Microscope (SEM) and bonding of both before and after acid treatment clay is studied by Fourier Transfer Infrared (FTIR) tool. Cation Exchange Capacity (CEC) measurement is carried out for the clay before acid treatment and after acid treatment.
KEYWORDS:
Bentonite Clay; Structure; Morphology; Bonding; Acid treatment
Copy the following to cite this article:
Pande S, Lagashetty A. K, Madhusoodana C. D, Venkataramana A. Acid Activation and Its Characterisation of Gulbarga City Bentonite Clay. Mat.Sci.Res.India;8(2)
|
Copy the following to cite this URL:
Pande S, Lagashetty A. K, Madhusoodana C. D, Venkataramana A. Acid Activation and Its Characterisation of Gulbarga City Bentonite Clay. Mat.Sci.Res.India;8(2). Available from: http://www.materialsciencejournal.org/?p=2650
|
Introduction
Clays commonly refer to either rocks that may consolidate or unconsolidated or a group of minerals having unique properties The clay mineral refers to polysilicate mineral & to mineral which impart plasticity to clay & which harden upon drying or firing. basic clay mineral of clays are montmorillonite or smectite usually are referred as bentonites. These bentonite clays finds number of applications catalyst and in the production of corbon free papers.1-2
Traditionally clays (rocks) are distinctive in at least two properties which render then technologically useful.3 Plasticity property signifies the property of the clay when wetted that permits deformation by application of relatively slight pressure & retention of the deformed shape after release of the pressure. Clay is predominantly composed of hydrous silicates of Al, Mg, K & Fe clay minerals are extremely fine particles and these are minerals which are layered silicates that are formed usually as products of chemical weathering of other silicate mineral at the earth’s surface.4-6 The physical observation, layered structure and other properties of clays and hard rocks distinguishes each other.
The clay mineral is the weathered product of magic silicates and is stable in temperature of the climates.7 It was known formerly as montmorillonite. Smectite has the ability to adsorb large amounts of water forming as water tight barrier. It is used extensively in the oil drilling industry and used by environmental engineers. The color of smectites can vary from white to brown or brownish green to blue green. This group composed of several mineral including pyrophyllite, talc, vermiculite, saponite, nontronite & montmorillonite they differ mostly in chemical content. Smectite is a 2:1 layer silicate, kaolinite which is a 1:1 layer silicate. Smectite has two silica tetrahedral sheets joined to a central octahedral sheet.8 Acid activation of the raw clay varies some of the properties and nature of the raw clay, such as chemical composition, cation exchange capacity, surface area etc.9
The present study reports collection bentonite raw clay from Gulbarga district and analyzed its composition by chemical method. The raw clay is made acid activation using nitric acid, Sulphuric acid and phosphoric acid respectively. X-ray diffraction study (XRD) tool is used to know structural behavior of the Bentonite clay before and after acid treatment. Scanning Electron Microscope (SEM) tool is helped to know morphology of the sulphuric acid treated clay. The bonding nature sample before and after acid treatment clay is studied by Fourier Transfer Infrared (FTIR) tool. Cation Exchange Capacity (CEC) measurement is carried out for the clay before acid treatment and after acid treatment.
Experimental
Materials and Methods
All chemicals used in the experiment were of AR grade and double distilled water. The processing clay sample is collected from Gulbarga district and operations like clay crushing, grinding and air-drying are carried out. Acid activation of clay is carried out in round bottom pyrex flask. The chemical analysis of the sample is carried out by literature method.
Physical Treatment of Raw Clay
The collected bentonite clay from locally available Gulbarga unit is obtained in the form of lumps. This is fed into ball mill with 100 alumina balls & milling was carried out of about 10 minutes. The raw clay obtained after milling was screened in the sieve shaker to get fine particles.
Acid Activation of Bentonite Clay
The acid activation experiment were carried out in a laboratory size stirred bath reactor. 40gm of raw clay sample is taken in three different round bottom pyrex flasks. 11ml, 12ml and 14ml of sulphuric acid, phosphoric acid and nitric acid is taken in first, second and third pyrex flasks respectively. All three flasks are refluxed for six hours under water bath. After refluxing stir the content of the flasks under magnetic stirrer in high speed for one hour. Cool the solution for room temperature and filter it by using Whatman 41 filter paper. Wash the sample with repeatedly with acetone and dried. The dried acid activated clay sample was ground well in a pestel and morter to reduce the size of the sample.
Cation Exchange Capacity (CEC)
CECs, defined as the milliequivalents of exchangeable cations contained in 100g of clay or clay minerals. This is an important property of the clay and has great influence on their technological applications, because they are fundamental control factor of the physical and chemical properties of the clays. This CEC were determined by the methylene blue standard procedure.10
Surface Area Determination
Scars method is used for determination of surface area of the raw clay and acid activated. Initially, 0.5g of bentonite clay treated with 0.05N hydrochloric acid to a pH 4-5 and 10g of sodium chloride is added to the 50ml volumetric flask and is diluted up to up to the mark with distilled water. The titration was carriedout with 0.05M sodium hydroxide in thermostatic bath at 298K. The volume (V) required to raise the pH from 3.5 to 9 was noted and the surface area was calculated by using following equation.
Surface area (m2/g) = 32V-25
Characterization
The X-ray diffraction patterns were obtained employing a Geol JDX-8p spectrometer using CuKα radiation. The X-rays generator was operated at 30kV and 20mA. The scanning range, 2θ/θ was selected. The scanning speed =10 min-1 were employed for precise lattice parameter determination. The shape, size, distribution of the powder and microstructure of the clay sample have examined using a Leica-440 Cambridge Stereoscan, scanning electron microscope tool. The SEM was operated at 20kV. The sample was made conducting by the sputtering of gold using a Poloron DC “sputtering unit” operated at 1.4kV and 18-20mA. The infrared spectra of the clay sample were recorded on a Perkin–Elmer FTIR spectrophotometer [Model 1000] in the range 300 cm-1 to 4000cm-1.
Results and Discussion
X-ray Diffraction Study
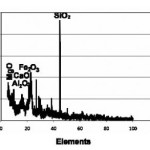
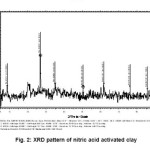
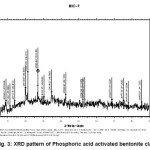
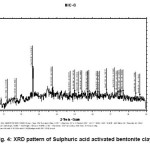
Figure-1 shows XRD pattern of raw bentonite clay. The pattern shows the presence of intensed peaks of some metals in the form its oxides, which is in agreement with chemical composition obtained by chemical analysis and is given in the table-1. The highly intenced peaks in the pattern confirms the crystalline behavior of the raw clay sample. Figure-2, 3 and 4 shows XRD pattern of acid activated clay sample by nitric acid, phosphoric acid and suplphuric acid respectively. These XRD patterns show sharp and highly intensed peaks indicates the more crystalline nature and less in particle size compared to the raw clay. The phase of the raw clay is changed to some extent depending upon the efficiency of the acids.
Figure 1: XRD pattern of raw bentonite clay
Table 1: Chemical Composition of bentonite raw clay
Figure 2: XRD pattern of nitric acid activated clay
Figure 3: XRD pattern of Phosphoric acid activated bentonite clay
Figure 4: XRD pattern of Sulphuric acid activated bentonite clay
Scanning Electron Microscope
Figure 5 shows SEM image of sulphuric acid activated bentonite clay. The image shows irregular shape of the particles with more compact arrangement with netting. Some small needled pores in the image may indicate high surface area and hence it may acts as good adsorbent.
Figure 5: SEM image of sulphuric acid activated bentonite clay
Infrared Study
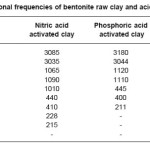
Table-2 gives the vibrational frequencies of raw clay and acids activated bentonite clay. The care full observation of the table, all these sample shows a broad peak at around 3000 cm-1 corresponds to water of adsorption and the peak at around 1000cm-1 to 1100 cm-1 is due to the presence of some overtones. The peaks 500cm-1 correspond to the metal-oxygen vibrational modes of the spinal compound.8 Some peaks at around 200 cm-1 is also observed is due to metal-metal vibration frequency range.
Table 2: Vibrational frequencies of bentonite raw clay and acid activated clay
Surface Area Determination
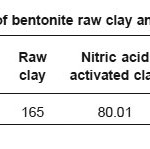
Table-3 shows surface area determination data of bentonite clay and acid activated clay. Careful observation of the table data, it is clearly indicating that, the surface area of acid activated clays is increased as compared to the raw clay. This table also shows the variation of surface area of different acids used for activation. The sulphuric acid shows highest surface area compared to the other two acids.
Table 3: Surface area of bentonite raw clay and acid activated clay
Cation Exchange Capacity
Table-4 shows CEC results obtained by methylene blue standard procedure. The table shows CEC values are decreasing compared to raw clay. The Phosphoric acid activated clay shows highest CEC value compared to other two acid activated clays.
Table 4: CEC values of bentonite raw clay and acid activated clay
Conclusions
This study gives the useful information that, the acid activated clays show the enhanced physical and chemical properties. The structural difference of raw clay and acid activated clay is well studied by XRD. This change may be removal of some impurity and reduction in the particle size and directs high applications as good adsorbent. Infrared study highlights the metal-oxygen bond and metal-metal bond in raw clay and acid activated clay. Detailed characterization and some studies of the raw clay and acid activated clays is our future direction of the work
Acknowledgements
Authors are thankful to Department of Science and Technology(DST), India (GrantNo.SR/ S1/PC-10/2005) New delhi and UGC Innovative Programme in Materials Chemistry, New Delhi(D.O.No. F.14-4/2001 (Innov. Policy/ ASIST) for their financial assistance.
References
- Kooli, F., Jones,W., Clay Sci., 6: 59 (1991).
CrossRef
- Adams, J.M., Appl.Clay Sci., 2: 309 (1978).
CrossRef
- parks, D.L., Environmental Soil Chemistry, Acdemic Press, Inc, California, (1995).
- Onal, M, Sarikaya, Y., Alemdarroglu, T and Bozdogan, I., Turk. J Chem, 26: 409 (2002)
- Madejova, J, Vibrational Spectroscopy, 31:1 (2003)
CrossRef
- Pande,S. Bedre, M..D, Madhusoodana, C.D. and Venktaramana, A., Mater. Sci.: An Indian Journal,(In Press)
- Ravichandran, J. Sivasankar, B., Clays and Clay Miner., 45: 854 (1997).
CrossRef
- Sankaya, Y, Sevinc, I and Akmc, M., Powder Tech., 116: 109 (2001).
CrossRef
- Gregg, S. and Sing, K. S. W., Adsorption, Surface Area and Porosity, Academic Press, London, (1982).
- Rytwo, G, Serban,C, Nir, S and Margulies, L., Clays Clay Miner. 39: 551 (1991).
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal