Anuraag Boddupalli1*, Ved Varun Aggarwal2, B. D. Malhotra2 And Radha Prasanna3
1Manipal Institute of Technology. Manipal - 576 104, India.
2Biomolecular Electronics and Conducting Polymer Research Group, Materials Physics and Engineering Division, National Physical Laboratory, New Delhi - 110 007, India.
3Division of Microbiology, Indian Agricultural Research Institute (IARI), New Delhi - 110 012, India.
DOI : http://dx.doi.org/10.13005/msri/080219
Article Publishing History
Article Received on : 10 Nov 2011
Article Accepted on : 28 Dec 2011
Article Published :
Plagiarism Check: No
Article Metrics
ABSTRACT:
The need for rapid evaluation of chemicals in the environment or human body has prompted researchers to develop easy to use biological assays or biosensors. The new developments in light sensitive and conductive polymers integrated with micro-optics can monitor “practically anything“ such as toxicity to DNA, life (cytotoxicity), endocrine disrupting chemicals or environmental pollutants. Such new generation biochips offer a panoramic view of once unthinkable diagnostics, propelling the laboratory into nanoscale dimensions.
KEYWORDS:
Bioreporters; Chemicals; Detection; Diagnostic
Copy the following to cite this article:
Boddupalli A, Aggarwal V. V, Malhotra B. D, Prasanna R. Biosensors: The Future of Chemical Diagnostics. Mat.Sci.Res.India;8(2)
|
Copy the following to cite this URL:
Boddupalli A, Aggarwal V. V, Malhotra B. D, Prasanna R. Biosensors: The Future of Chemical Diagnostics. Mat.Sci.Res.India;8(2). Available from: http://www.materialsciencejournal.org/?p=2671
|
Introduction
The development of biological sensors, or biosensors, is a rapidly growing field and scientists in areas as diverse as chemistry, biotechnology, mechanical engineering and electrical engineering are working to create biosensors that have medical and industrial applications.1 These researchers employ technologies as cutting edge as stretchable electronics and as simple dipstick tests, using materials as exotic as silk in ancient times to gold nanoparticles in the 21st Century. Futuristic applications include creating biosensors that could to monitor the heart of a patient with arrhythmia, detect toxins such as lead in the home or laboratory, or be used in futuristic brain-machine interfaces.2
With the vast and diverse applications emerging for biosensors, there are several reasons why it has become of paramount importance to understand and design different types for both qualitative and quantitative detection. In the modern day world, where the percent population suffering from carcinogen intake is rising alarmingly and environmental awareness growing, biosensors are becoming important tools for diagnostics3. Most doctors rely on testing to confirm their diagnosis, not going by just the external symptoms to avoid giving excess or wrong medication which would endanger the life of the patient. Added to it is the ease of administration of biosensors to any process or medical condition, also giving accurate and fast responses are few of the many reasons for the need of biosensors. However, biosensors can be brought in for production only when they are completely economically viable and cheap, ensuring that advancement in technology is not burdensome on the common man’s pocket.
What is a Biosensor?
A Biosensor is an analytical device for the detection of an analyte that combines a biological component with a physicochemical detector component. It consists of 3 parts:
The analyte /chemical compound
The sensitive biological element (biological material (e.g. tissue, microorganisms, organelles, cell receptors, enzymes, antibodies etc., a biologically derived material or biomimic-which imitates the natural signal of, etc.) that transforms the signal resulting from the interaction of the analyte with the biological element into another signal (i.e., transducers) that can be more easily measured and quantified;
Associated electronics or signal processors that are primarily responsible for the display of the results in a user-friendly way. This sometimes accounts for the most expensive part of the sensor device; however, it is possible to generate a user friendly display that includes transducer and sensitive element.
A common example of a commercial biosensor is the blood glucose biosensor, which uses the enzyme glucose oxidase to breakdown blood glucose. The first step involves oxidization of glucose using two electrons to reduce the Flavinine Adenosine Diphosphate (a component of the enzyme) to FADH2 (Flavinine Adenosine Dihydrate). This in turn is oxidized by the electrode (accepting two electrons from the electrode) in a number of steps. The resulting current is a measure of the concentration of glucose. In this case, the electrode is the transducer and the enzyme is the biologically active component. The Glucose sensor was first developed by Clark in 1962. And soon several pharmaceutical companies were able to commercialize the process and develop ready to use devices.
The Basic Characteristics of a Biosensor are
Linearity: Maximum linear value of the sensor calibration curve. Linearity of the sensor must be high for the detection of high substrate concentration.
Sensitivity: The value of the electrode response per substrate concentration.
Selectivity: Interference of chemicals must be minimized for obtaining the correct result.
Response Time: The necessary time for having 95% of the response.
As they are such a versatile tool, many methods have been devised for detection of signal from the system. Some of them are given below:
Fluorescence
DNA Microarray
SPR-Surface plasmon resonance
Impedance spectroscopy
SPM (Scanning probe microscopy, AFM, STM)
QCM (Quartz crystal microbalance)
SERS (Surface Enhanced Raman Spectroscopy)
Electrochemical Detection
Among these methods, the following methods are of more recent origin include surface plasmon resonance (SPR) and Surface enhanced Raman spectroscopy or surface enhanced Raman scattering (SERS).The excitation of surface plasmons by light is denoted as a surface plasmon resonance (SPR) for planar surfaces or localized surface plasmon resonance (LSPR) for nanometer-sized metallic structures. This phenomenon is the basis of many standard tools for measuring adsorption of material onto planar metal (typically gold and silver) surfaces or onto the surface of metal nanoparticles. It is the fundamental principle behind many color based biosensor applications and different lab-on-a-chip sensors.
A lab-on-a-chip (LOC) is a device that integrates one or several laboratory functions on a single chip of only millimeters to a few square centimeters in size. LOCs deal with the handling of extremely small fluid volumes down to less than picolitres. Lab-on-a-chip devices are a subset of microelectromechanical (MEMS) devices and often indicated by “Micro Total Analysis Systems” (¼TAS) as well. Microfluidics is a broader term that describes also mechanical flow control devices like pumps and valves or sensors like flow meters and viscometers. However, strictly regarded “Lab-on-a-Chip” indicates generally the scaling of single or multiple lab processes down to chip-format, whereas “µTAS is dedicated to the integration of the total sequence of lab processes to perform chemical analysis. The term “Lab-on-a-Chip” was introduced later on when it turned out that µTAS technologies were more widely applicable than only for analysis purposes.
Surface enhanced Raman spectroscopy or surface enhanced Raman scattering (SERS) is a surface-sensitive technique that enhances Raman scattering by molecules adsorbed on rough metal surfaces.4,5 The enhancement factor can be as much as 1010 to 1011, which refelects the sensitivity of the technique in detecting single molecules.6,7 Raman scattering or the Raman Effect is the inelastic scattering of a photon. It was discovered by Sir C.V. Raman and K.S. Krishnan in liquids; and by Grigory Landsberg and Leonid Mandelstam in crystals. When light is scattered from an atom or molecule, most photons are elastically scattered (Rayleigh scattering), such that the scattered photons have the same energy or frequency and wavelength as the incident photons. However, a small fraction of the scattered light (approximately 1 in 10 million photons) is scattered by an excitation, with the scattered photons having a frequency different from, and usually lower than, the frequency of the incident photons. In a gas, Raman scattering can occur with a change in vibrational, rotational or electronic energy of a molecule.
Biosensors can have Applications in Diverse Fields
Study of biomolecules and how they interact with one another
Drug Development
In- home medical diagnosis
Environmental field monitoring
Scientific crime detection
Quality control in small food factory
Food Analysis
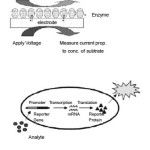
The latest development in the field of biosensors has been the research being done on Bioreporters.6-8 Bioreporters refer to intact, living microbial cells that have been genetically engineered to produce a measurable signal in response to a specific chemical or physical agent in their environment. Bioreporters contain two essential genetic elements, a promoter gene and a reporter gene. The promoter gene is turned on (transcribed) when the target agent is present in the cell’s environment. The promoter gene in a normal bacterial cell is linked to other genes that are then likewise transcribed and then translated into proteins that help the cell in either combating or adapting to the agent to which it has been exposed. In the case of a bioreporter, these genes, or portions thereof, have been removed and replaced with a reporter gene. Consequently, turning on the promoter gene now causes the reporter gene to be turned on. Activation of the reporter gene leads to production of reporter proteins that ultimately generate some type of a detectable signal. Therefore, the presence of a signal indicates that the bioreporter has sensed a particular target agent in its environment.
Below are some examples of Bioreporter compounds and their mode of action
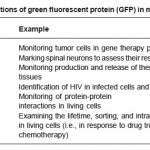
Table 1: Applications of green fluorescent protein (GFP) in mammalian cells
Firefly Luciferase (Luc)
Firefly luciferase catalyzes a reaction that produces visible light in the 550–575 nm range. A click-beetle luciferase is also available that produces light at a peak closer to 595 nm. Both luciferases require the addition of an exogenous substrate (luciferin) for the light reaction to occur. Numerous luc-based bioreporters have been constructed for the detection of a wide array of inorganic and organic compounds of environmental concern. Their most promising application, however, probably lies within the field of medical diagnostics. Insertion of the luc genes into a human cervical carcinoma cell line (HeLa) illustrated that tumorcell clearance could be visualized within a living mouse by simply scanning with a chargecoupled device camera, allowing for chemotherapy treatment to rapidly be monitored on-line and in real-time.8-10 In another example, the luc genes were inserted into human breast cancer cell lines to develop a bioassay for the detection and measurement of substances with potential estrogenic and anti-estrogenic activity.
Aequorin
Aequorin is a photoprotein isolated from the bioluminescent jellyfish Aequorea victoria. Upon addition of calcium ions (Ca2+) and coelenterazine, a reaction occurs whose end result is the generation of blue light in the 460-470 nm range. Aequorin has been incorporated into human B cell lines for the detection of pathogenic bacteria and viruses in what is referred to as the CANARY assay (Cellular Analysis and Notification of Antigen Risks and Yields).9 The B cells are genetically engineered to produce aequorin. Upon exposure to antigens of different pathogens, the recombinant B cells emit light as a result of activation of an intracellular signaling cascade that releases calcium ions inside the cell.
Green Fluorescent Protein (GFP)
Green fluorescent protein (GFP) is also a photoprotein isolated and cloned from the jellyfish Aequorea victoria.12-14 Variants have also been isolated from the sea pansy Renilla reniformis. GFP, like aequorin, produces a blue fluorescent signal, but without the required addition of an exogenous substrate. All that is required is an ultraviolet light source to activate the fluorescent properties of the photoprotein. This ability to autofluoresce makes GFP highly desirable in biosensing assays since it can be used on-line and in real-time to monitor intact, living cells. Additionally, the ability to alter GFP to produce light emissions besides blue (i.e., cyan, red, and yellow) allows it to be used as a multianalyte detector. Consequently, GFP has been used extensively in bioreporter constructs within bacterial, yeast, nematode, plant, and mammalian hosts. Table 3 lists some examples of GFP applications in mammalian cell systems, where its use has revolutionized much of what we understand about the dynamics of cytoplasmic, cytoskeletal, and organellar proteins and their intracellular interactions.
Uroporphyrinogen (Urogen) III Methyltransferase (UMT)
UMT catalyzes a reaction that yields two fluorescent products which produce a red-orange fluorescence in the 590-770 nm range when illuminated with ultraviolet light.11 So as with GFP, no addition of exogenous substrates is required. UMT has been used as a bioreporter for the selection of recombinant plasmids, as a marker for gene transcription in bacterial, yeast, and mammalian cells, and for the detection of toxic salts such as arsenite and antimonite.
Figure 1
Benefits of Using Bioreporters
Bioreporter technology will provide a robust, cost-effective, quantitative method for rapid and selective detection and monitoring of chemical and biological agents in applications as far ranging as medical diagnostics, precision agriculture, environmental monitoring, food safety, and process monitoring and control.1,2,12-13 Their attractiveness lies in the fact that they can often be implemented in real-time, on-line bioassays within intact, living cell systems, thus providing a unique and revolutionarily new perspective on bacterial, plant, and mammalian physiology and intracellular interactions.15-17 In conjunction with advanced photonic detection technologies such as the BBIC, bioreporters are increasingly becoming important tools for noninvasive monitoring regimes, especially in animal model systems. The monitoring of light requires less time and fewer animals than conventional methods, thus reducing the cost of obtaining biologically relevant data. Consequently, the study of infectious disease, tumor progression and metastasis, gene therapy, mammalian development, and many other areas in which animal models are used as predictors for the human response to therapy can be greatly simplified and accelerated.18,19 The same ideals apply in cases of environmental monitoring and food safety, where rapid and remote monitoring using BBIC devices can strategically inpoint areas of biological hazard, whether in the form of biological warfare agents or pathogenic E. coli presence. Further advances in bioreporter genetics and miniaturized optics will clearly impact future monitoring and detection strategies in these fields as well as a host of others.
References
- Malhotra BD, Singhal R, Chaubey A, Sharma SK, Kumar A. Curr Appl Phys 5: 92 (2005).
CrossRef
- Pandey P, Arya, SK, Malhotra BD, Nanomaterials for biosensor applications Encyclopedia of Nanoscience and Nanotechnology, American Scientific Publishers (2008).
- Malhotra BD Chaubey A, Sensors Actuators B: Chemical 91: 117 (2003).
CrossRef
- Blackie EJ, Le Ru, Eric C, Etchegoin Pablo G, J Am Chem Soc 131: 14466 (2009).
CrossRef
- Blackie EJ, Le Ru, Eric C, Meyer M, Etchegoin PG, J Phys ChemC 111: 13794 (2007).
- Davidov, Y. et al., Mutation Res 466: 97 (2000).
CrossRef
- Gerard M, Chaubey A. Malhotra BD, Application of conducting polymers to biosensors. Biosensors Bioelectronics 17: 345-359 (2002).
CrossRef
- Hansen LH, Sorensen SJ, Detection and quantification of tetracyclines by whole cell biosensors. FEMS Microbiol Lett 190: 273 (2000).
CrossRef
- HERMENS J. et al. Ecotoxicol Environ Saf 9:17 (1985).
CrossRef
- Hutter E, Fendler J, Adv Mater 19: 1685 (2004).
CrossRef
- King JMH et al., Science, 249, 778-781 (1990).
CrossRef
- Le Ru, Eric C, Meyer M, Etchegoin PG, J Phys Chem B 110: 1944 (2006).
CrossRef
- Matharu Z, Pandey P, Pandey MK, Gupta V, Malhotra BD. Electroanalysis 21 :1587 (2009).
CrossRef
- Maier SS, Plasmonics: Fundamentals and Applications. Springer (2007).
CrossRef
- Meighen EA, Genetics of bacterial bioluminescence. Annu Rev Genet 28: 117 (1994).
CrossRef
- Nie S, Emory SR, Science 275: 1102 (1997).
CrossRef
- Pandey P, Arya SK, Matharu Z, Singh SP, Datta M, Malhotra B D, J Appl Polymer Sci 110: 988 (2008).
CrossRef
- Raether H, Surface plasmons on smooth and rough surfaces and on gratings. Springer Verlag, Berlin (1988)
CrossRef
- Schasfoort, R.B.M. and Tudos, A. J (Eds.) Handbook of Surface Plasmon Resonance. RSC Publ (2008).
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal