Ranjita Mandal1,2*, V. N. Bhoraskar2 and D. Sengupta1
1Department of Geology and Geophysics, Indian Institute of Technology, Kharagpur - 721 302, India.
2Department of Physics, University of Pune, Pune - 411 007 India.
DOI : http://dx.doi.org/10.13005/msri/080226
Article Publishing History
Article Received on : 01 Oct 2011
Article Accepted on : 18 Nov 2011
Article Published :
Plagiarism Check: No
Article Metrics
ABSTRACT:
Teflon is a polymer material and acts as an electrical insulator. But when doped with alkali metals like lithium and sodium, it has been found that there is a decrease in resistivity of the samples. A simple method has been described here to dope the Teflon chemically and observe the resistivity changes.
KEYWORDS:
Resistivity; Teflon; Alkali metals; Tetrahydrofuran; Activation analysis
Copy the following to cite this article:
Mandal R, Bhoraskar V. N, Sengupta D. Resistivity Measurement of Teflon Doped With Sodium and Lithium. Mat.Sci.Res.India;8(2)
|
Copy the following to cite this URL:
Mandal R, Bhoraskar V. N, Sengupta D. Resistivity Measurement of Teflon Doped With Sodium and Lithium. Mat.Sci.Res.India;8(2). Available from: http://www.materialsciencejournal.org/?p=2699
|
Introduction
Nowadays, considerable amount of work is being carried out in the field of polymers and many workers are trying to obtain good quality conducting layer on a polymer. Alkali metals Lithium and Sodium are elements which when doped in a polymer changes the surface resistivity. Chemical methods are used to dope these metals on polymers.
Material and Methods
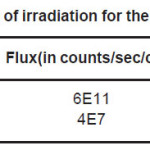
A Teflon sheet of 2mm thickness was cut in pieces of area 1cm2 and were cleaned using chemical treatment. These pieces were irradiated before and in some instances after the doping of sample with Lithium or Sodium in order to see the effect of irradiation on the resistivity of the samples with the following sources as shown in Table 1. The irradiation was carried out for duration enough to get good fluence.
Table 1: Sources of irradiation for the Teflon samples
A solution1,2 was prepared to dope the samples with Lithium or Sodium. This solution was prepared by taking 100ml of dry Tetrahydrofuran (THF) and adding 0.05mol(6.4gm) of Napthalene.
After this 0.1mol of either Lithium or Sodium is taken and stirred in the naphthalene added Tetrahydrofuran solution. A dark greenish blue solution is formed. This solution of Lithium or Sodium is taken and the unirradiated and pre-irradiated Teflon samples are dipped into it and taken out after 1, 2, 3, 4 and 5 days in small batches of four samples of each group.
The amount of Sodium or Lithium doped on the sample was estimated by weighing the sample before and after dipping in the Sodium or Lithium solution. The amount of sodium was also estimated by the activation analysis[3,4,5,6] using the reaction
Na23(n,p)Ne23 with t1/2= 37 seconds and Eγ=0.44 MeV(100%)
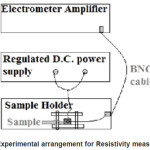
The resistivity measurement was done by a simple arrangement using a electrometer amplifier, a regulated D.C. power supply and a sample holder as shown in figure 1. The external voltage(Vext) was given from the D.C. Supply of about 10V and the internal voltage(Vint) was shown on the electrometer. The input resistance (Rint) was applied of about 1012 Ohms and observed on the electrometer. The Current(I) could be given by Vint/Rint or Vext/Rsample and this way the Rsample can be obtained. And the resistivity(ρ) was given as
ρ= Rsample(l/A)
where l and A is the thickness and area of sample. In case of some of the Sodium diffused samples, the current through the sample was in the order of micrometers and so could not be measured using the electrometer in the present setup.
Figure 1: Experimental arrangement for Resistivity measurement
Results and Discussions
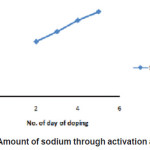
The activity of Sodium obtained through activation analysis is shown in Figure 2
Figure 2: Amount of sodium through activation analysis
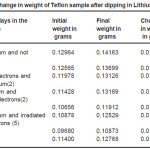
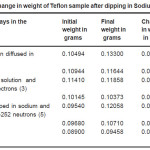
This figure shows that with the increase in the number of days of dipping the Teflon sample in the sodium solution, the amount of sodium doped on Teflon sample increases. This indicates that diffusion of sodium increases when kept in contact with the Sodium solution. The results obtained on amount of Lithium or Sodium doped on Teflon is shown in Table 2 and 3 respectively. The results show that there is a change in weight which indicates that the Teflon samples do get doped by the lithium and sodium solution and the variation shows an increase in the weight with increase in number of days dipped in the solution. Further, for the lithium doped samples, the increase in weight is more for irradiated samples.
Table 2: Change in weight of Teflon sample after dipping in Lithium solution
Table 3: Change in weight of Teflon sample after dipping in Sodium solution
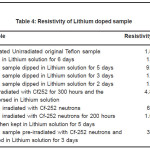
The results for the resistivity values obtained are shown in Table 4 for Lithium doped samples and Table 5 for Sodium doped samples. The results shows a decrease in resistivity for all the doped samples especially the unirradiated samples. Further, the resistivity values show a decreasing trend with increase in the doping for both Sodium and Lithium. The irradiated samples, however, show a higher resistivity than the unirradiated lithium doped samples. The pre-irradiated sample shows a lower resistivity than the post-irradiated sample.
Table 4: Resistivity of Lithium doped sample
Table 5: Resistivity of sodium doped samples
I thank S.J.Deepa for her help in preparation of samples. I also thank Dr. Dhavale and his students of Chemistry department, Pune university for their help while working in their laboratory for preparing the Tetrahydrofuran solution. I am thankful to Department of Science and Technology and Council of Sceintific and Industrial Research for their financial support.
References
- J. R. Nowers and B. Narasimhan, Polymer, 47: 1108-1118 (2006).
CrossRef
- Katsumi Yoshino, Shozo Yanagida, Toshihiko Sakai, Takayuki Azuma, Yoshio Iniushi and Hiroshi Sakura, Jpn. J. Appl. Phys. , 21: pp. L301-L302 (1982).
CrossRef
- V.N. Bhoraskar, Ind. J. Pure and Appl. Phys., 27: 648 (1989).
- L.F. Curtiss, An introduction to neutron physics, D. Van Nostrand Company, Inc.,Princeton, New Jersey, (1959) .
- Technical report series no.273, Handbook on nuclear activation data by international atomic energy agency.EXFOR Nuclear Reaction Data, http://www.nds.iaea.org/exfor

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal