Study of Process Parameters in Reduction from Doped Tungsten Blue Oxide to Non-Sag Tungsten Powder
V. Shantha1*, M. N. Vijayshankar2 and D. N. Drakshayani3
1Department of Mechanical Engineering, Sir MVIT, Bangalore - 562 157, India.
2Avasarala Technologies Limited, Mysore - 570 017, India.
3Department of Mechanical Engineering, Sir MVIT, Bangalore - 562 157, India.
DOI : http://dx.doi.org/10.13005/msri/080220
Article Publishing History
Article Received on : 20 Mar 2011
Article Accepted on : 7 May 2011
Article Published :
Plagiarism Check: No
Article Metrics
ABSTRACT:
The powders used for non-sag tungsten manufacturing are produced by hydrogen reduction. Dopants (Aluminium, Potassium & Silicon) are added to the Tungsten blue oxide to impart non-sag properties. The non-sag (NS) effect of the dopant can be achieved only when a certain amount of potassium-containing phase is incorporated into the metal and is optimally dispersed. The particle size and potassium (K) content of the NS tungsten powder is affected by reduction conditions. In the present work the effect of reduction parameters like Temperature & Push Time on Average Particle Size & Potassium content of washed & dried NS tungsten powder are presented. The results indicated that with increase in Temperature and Push time the average particle size increases and potassium content in the powder decreases. An attempt has been made to correlate these and optimize the reduction conditions.
KEYWORDS:
Doped tungsten; Tungsten blue oxide; NS tungsten powder; Hydrogen reduction
Copy the following to cite this article:
Shantha V, Vijayshankar M. N, Drakshayani D. N. Study of Process Parameters in Reduction from Doped Tungsten Blue Oxide to Non-Sag Tungsten Powder. Mat.Sci.Res.India;8(2)
|
Copy the following to cite this URL:
Shantha V, Vijayshankar M. N, Drakshayani D. N. Study of Process Parameters in Reduction from Doped Tungsten Blue Oxide to Non-Sag Tungsten Powder. Mat.Sci.Res.India;8(2). Available from: http://www.materialsciencejournal.org/?p=2674
|
Introduction
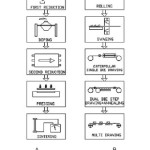
Tungsten has the highest melting point and lowest vapour pressure amongst all metals. In addition, it has excellent high temperature strength and hardness of the four common refractory metals. These unique combinations of properties are tailor-made for certain applications. One such application is for the manufacture of filaments. Tungsten lamp filaments must operate at high temperature for long times without significant distortion of the initial coil geometry. To meet these requirements, a bubble strengthening method is applied by a special alloying procedure called AKS-doping referring to the three dope elements of Aluminium (Al), Potassium(K) and Silicon(Si).1 The non-sag tungsten is usually produced via conventional powder metallurgical process2 (Fig. 1). It is the only process that readily affords critical control over the content and dispersion of the dopant elements, which generate special non-sag characteristics.
The starting material for the production of non-sag tungsten is Ammonium Para Tungstate (APT). The reduction from APT to metal powder involves three stages. In the first stage APT is reduced to Tungsten Blue Oxide (TBO). In the second stage the Dopants (K,Al & Si) are added to the Tungsten blue oxide to impart non-sag properties and in the third stage Doped Tungsten Blue Oxide is reduced to tungsten metal powder. Potassium is virtually insoluble in tungsten, and it remains in the sintered ingot in small, roughly spherical pores.3-5 About 10% of the doping potassium is necessary in the tungsten metal to produce the non-sag effect in bubble form. Potassium bubbles are required to produce the interlocking grain structure that gives the tungsten lamp filament a long life.6 Potassium is very effective in preventing deformation at operational temperatures of 32000C.7 During the deformation of the sintered ingots the spherical bubbles change their morphology into elongated bubble tubes or ellipsoids. Silicon is necessary to achieve sufficient potassium entrapment during reduction of doped blue oxide by keeping the oxide bed loose & permeable.8,9 Aluminium helps in superior NS quality of the tungsten wire.
The reaction from APT ((NH4)10W12O41.5H2O) to Tungsten (W).10
(NH4)10W12O41.5H2O → 12 WO3 + 10 NH3 + 10 H2O … (1)
4WO3 + H2 → W4O11 + H2 …(2)
1/3 W4O11 + H2 → 4/3 WO2 + H2O … (3)
1/2 WO2 + H2 → 1/2 W + H2O …(4)
The specific goal of the NS powder manufacture is to produce a tungsten powder with an average particle size in the range of 3-5µm (which is the optimal grain size for powder metallurgical processing) and 100-160 ppm of Potassium.11
Figure 1: Schematic process of tungsten wire production : (A) Chemical reduction, doping, pressing and sintering of tungsten powder. (B) Rolling, swaging and wire drawing of the sintered tungsten ingot
Experimental Set Up and Procedure
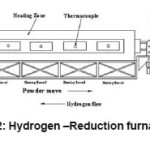
Doped tungsten blue oxide was used for the investigations. Reduction process is done in push type tube furnace. A tube furnace consists of several tubes made of inconel or heat resistant alloy. The furnace is electrically heated and has four heating zones. Each heating zone temperature is controlled independently. The ends of the tube are fitted with a seal which can be opened for boat loading and emptying, and with entrance and exit ports for the hydrogen12 (Fig. 2). The boats, which carry the powder, are also made of a heat resistant alloy. The boats are filled with fixed amount of powder and then pushed through the furnace at regular intervals. The hydrogen flow is countercurrent to the tungsten movement so that the reduced oxide sees the driest hydrogen.10
There are two guiding factors in the reduction: Particle size and Potassium content.10 This article presents the effect of 4th Zone Temperature and Push Time during the reduction of doped tungsten blue oxide to NS tungsten powder on these guiding factors.
The reduction experiments were done in single–boat push-type furnaces, using hydrogen with a dew point of -700C. During tungsten blue oxide reduction water from the powder layer is a determining factor.13 Different parameters in reduction process are furnace temperature, boatload, hydrogen flow & push time. In our investigation, experiments were conducted to find Potassium content & Average Particle Size of the powder by varying- (i)Temperature in 4th zone & (ii) Push Time .
The Average Particle Size is measured by using Fisher Sub Sieve Sizer. To know the Potassium content “Varian Atomic Absorption Spectrometer is used and Type K: Chromel-Alumel Thermocouple is used to measure the furnace temperature.
Figure 2: Hydrogen–Reduction furnace
Experimental Results
Effect of Temperature in 4th zone on potassium content & Average Particle Size (APS) of the Tungsten Powder in Second reduction.
Process Parameters
|
APS of Blue Oxide =
|
8.6µm
|
|
Hydrogen flow rate =
|
110 lpm
|
|
Boat load =
|
300 gms.
|
|
Push Time =
|
20 minutes
|
|
Zone Temperature =
|
I
|
Zone – 7300C
|
|
|
II Zone – 7800C
|
|
|
III
|
Zone – 8300C
|
Effect of Push Time on potassium content & Average Particle Size (APS) of the Tungsten Powder in Second reduction.
Process Parameters
|
APS of Blue Oxide
|
8.6 µm
|
|
|
Hydrogen flow rate
|
110 lpm.
|
|
|
Boat load
|
300gms
|
|
|
Zone Temperature
|
I
|
Zone- 7300C
|
|
|
II Zone- 7800C
|
|
|
III
|
Zone-
|
8300C
|
Results and Discussion
After reduction nearly all the dopants added to the TBO appear to remain in the tungsten powder. However, only about 10% are actually incorporated within the tungsten particles and the rest are only on the particle surfaces. During subsequent leaching of the powder in Hydro Chloric (HCl) and Hydro fluoric(HF) acids, only the dopants trapped internally are retained, while those merely attached to the surface are washed.9
Effect of Temperature in 4th Zone
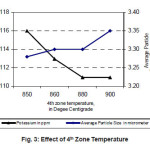
The most important parameter for determining the average particle size of the tungsten powders is the reduction temperature.9 In our experiment, it was decided to vary the 4th zone temperature as coarsening of powder takes place predominantly in this region. As 4th zone temperature increases Average particle size of the tungsten powder also increases.
When the reduction is carried out at high temperature, the specific surface area decreases steadily due to the fact that the primary particles formed at intermediate stages of reduction are large. The particle size of metallic tungsten after reduction is affected by primary particles at the intermediate stage of reduction of blue oxide. Reduction at higher temperature produce larger metal powders.12
And with increase in 4th zone temperature potassium content in the powder decreases. The dopants are relatively volatile so that at low temperatures the dopants are retained and are entrapped in the reduced metal powder. At high temperatures the dopants will be driven off before they become entrapped by the reduction to tungsten metal. Hence Potassium content decreases with increase in 4th Zone temperature.10
Figure 3: Effect of 4th Zone Temperature
Effect of Push Time
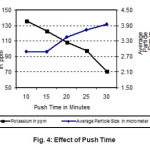
With increase in push time, the residence time of the powder in the 4th zone increases. This will increase the contact time between the powder and hydrogen flowing counter current to the feeding direction. As a result the interaction between the powder particles and moist hydrogen will be enhanced. Consequently, the particle size of the powder will increase. Similarly the potassium content of the powder will decrease due to the increase in particle size and also as hydrogen carries away more potassium due to increase in residence time of the powder inside the furnace.
Figure 4: Effect of Push Time
Water Cycle Reaction10
W + 4 H2O→WO3.H2O + 3 H2 …(5)
Conclusion
With increase in 4th zone temperature average particle size of the reduced tungsten powder increases because at high temperatures the particle size of metallic tungsten after reduction is affected by primary particles at the intermediate stage of reduction of blue oxide. Dopants are relatively volatile, at lower temperatures the dopants are retained and are entrapped in the reduced tungsten powder. At high temperatures the dopants will be driven off before they become entrapped by the reduction to tungsten metal and hence the potassium content in the reduced metal powder decreases.
With increase in push time average particle size of the reduced tungsten powder increases since interaction between the powder particles and moist hydrogen is enhanced and the potassium content of the powder decreases due to the increase in particle size and also as hydrogen carries away more potassium due to increase in residence time of the powder inside the furnace.
Acknowledgements
The present work was done at M/s. Avasarala Technologies Limited, Mysore, India. Authors would like to acknowledge M/s. Avasarala Technologies Limited, for their co-operation and assistance in this work.
References
- O. Horacsek, L. Bartha, Int. Jr. of Refractory Metals & Hard Materials, 22: pp. 9-15 (2004).
- G.Eichelbronner, Int. Jr. of Refractory Metals & Hard Materials, 16: pp. 5-11 (1998).
- J. L. Walter, K. A. Lou and M. R. Vukevich,Proc. 12th Int. Plansee Conf. H.Bildstein and R. M Ortner, eds. Verlagsanstalt Tyrolia, Innsbruck, Austria, 1: 493-512 (1989).
- B. P. Bewley, Proc. 5th Int. Tungsten Symp., MPR publishing, Shrewsbury, United Kingdom, 227-45 (1991).
- B. P. Bewley, N. Lewis, K. A Lou, Metll. Trans.A, 23A: 121-33 (1992).
- D. B. Snow, Met. Transactions, 5: 2375-2381 (1974).
- Andreas Hoffmann, Ingmar Wesemann, Proc.of International conference on Tungsten, Refractory and Hard Materials VIII, (2011).
- J.Neugebauer & L. Bartha, Int. Jr. of RefractoryMetals & Hard Materials, 13: pp.1-34, (1995).
- Wolf-Dieter Schubert, Benno Lux & Burghard Zeiler, Int. Jr. of Refractory Metals & Hard Materials, 13:.pp. 119-135, (1995).
- James A Mullendore., In The Metallurgy of Doped/Non-Sag Tungsten, ed. E. Pink & L.Bartha, Elsevier, London, pp.61-82, 1989 .
- Erik Lassner, Wolf-Dieter Schubert, Int. Jr. of Refractory Metals & Hard Materials, 13: pp.111-117, (1995).
- Hiroshi Yamamoto., In The Metallurgy of Doped/Non-Sag Tungsten, ed. E. Pink & L.Bartha, Elsevier, London, 31-45 (1989).
- B.Zeiler, W.D.Schubert & Lux, Int. Jr. of Refractory Metals & Hard Materials, 10: 91-105 (1991).

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal