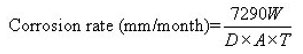Introduction
The austenitic stainless steels 300 series and their properties and general characteristics are important. These materials are used in advanced applications as gas turbine, nuclear power station, chemical, petrochemical, machinery, automobile and therefore a lot of research has been devoted to these materials.1 The cold rolling effect on stainless steels leads increased in dislocation density, vacancy, residual stresses and volume fraction of strain-induced α’-martensite phase in 304L SS was varied with the rolling conditions.2 During plastic deformation, depending on the steel composition, the cold working variables, and stacking faults, two different martensitic phase, ε martensite (HCP) and α’- martensite (BCC) can be formed.3
The α’ phase is often called strain-induced martensite because it is produced by a diffusion less phase transformation. The most probable way of the phase transformation in the 300 series austenitic stainless steels is the γ→ε→α or γ→α.4 The strain-induced transformation of austenite to α’-martensite phase and the evolution of texture in both the phases were studied as a function of rolling reduction as well as aging temperature in the metastable 304L austenitic stainless steel.5 Precipitation of sigma and chi phases was accelerated by cold rolling and they were observed at grain boundaries in lower cold-rolling. It was also seen in grain interiors in higher cold-rolling. The effects of prior cold rolling up to an 80 pct reduction in thickness on the sensitization desensitization behavior of type AISI 304 stainless steel and its susceptibility to intergranular corrosion have been studied by electrochemical Potentiokinetic reactivation (EPR) methods. The deformed type 304 stainless steel experienced desensitization at higher temperatures and times, and it was found to be enhanced by increased cold deformation.6 The sensitization of the austenitic stainless steels accelerated the precipitation of Cr23C6 in the austenitic matrix in the case of cold worked samples.7 In addition to good mechanicalproperties, corrosion resistant properties are equally importance. The atmospheric environments are classified as rural, urban, industrial, marine, or combinations of these.8-9 Atmospheric Corrosion Test is very important test to study the effect of industrial environment on cold rolled stainless steel.10 The environment has a big effect on the corrosion of stainless steel. The application of stainless steel in the atmosphere is mostly for decorative cladding in architecture. Slight corrosion will cause serious damage on the appearance of buildings. Therefore, the study of corrosion of stainless steels in the atmosphere has been another criterion in recent years 11 to evaluate corrosion resistance properties. Conventional atmospheric parameters that may lead to metal corrosion comprise of weathering factors such as temperature, moisture, rainfall, solar radiation, wind velocity etc. Air pollutants such as sulphur dioxide, hydrogen sulphide, oxides of nitrogen, chlorides have also been found to contribute to atmospheric corrosion.12 Pitting corrosion is the result of the local destruction of the passive film and subsequent corrosion of the steel. It generally occurs in chloride, halide or bromide solutions. The concentration of chloride ions in the vicinity of a pit can be thousands times greater than that the solution as a whole.13-14 In particular, special attention was given on the amount of martensite α’ phase retaining in the structure of tested stainless steels after cold rolling and also to study the atmospheric and immersion corrosion propertiesin non sensitized and sensitized samples at 600o C for 1 hour, 5 hours and 30 hours.
Experimental
Material Processing
Stainless steel AISI 304 having chemical composition of 0.05% C, 18.54% Cr, 9.8% Ni, 1.8% Mn, and 0.54% Si by weight was used. The plates were solution annealed at 1100OC for 1 hour to Homogenize and then cut into various plate sizes for cold rolling operation. The original thickness of the material was 10 mm. The stainless steel plates were processed for uniaxial cold rolling from 10-80% reduction in thickness. The samples were cut into blocks measuring 2.0 cm x 2.0 cm thickness from each cold rolled plate.
Determination of Deformation Induced Martensite (DIM)
For determination of deformation induced martensite, specimens of size 2.0 cm x 2.0 cm were polished up to 1000 grit emery paper and one micron Alumina paste and electro polished in a solution of 125 ml sulphuric acid (H2SO4) plus 650 ml orthophosphoric (H3PO4) acid in 225 ml distilled water. The electro polishing was performed at 80 ± 2OC under controlled current and potential conditions with a copper rod used as a cathode. Deformation induced martensite formed during cold rolling was determined by Schultz reflection Technique added X- ray diffraction analysis.
Double Loop Electrochemical Potentiokinetic Reactivation (DL-EPR) Test
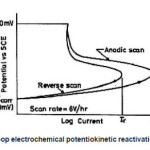
The double loop electrochemical Potentiokinetic reactivation technique (DL-EPR) was a technique which employed potentiodynamic scans to assess the degree of sensitization as shown in Fig.1. The tests were performed in a 0.5 M H2SO4 + 0.01 M KSCN solution at room temperature. Before polarizing, the samples were cathodically cleaned at a potential of -900 mV with respect to saturated calomel electrode (SCE) for 2 minutes and allowed to stabilize at open circuit potential rather than passivating the sample potentiostatically, the potential was scanned dynamically from open circuit (typically -100 mV SCE) up to +300 mV SCE (Point 2) at scan rate of 6 V/h, after that it was scanned back down to the original open circuit potential. The degree of sensitization (DOS) was evaluated by measuring the ratio of Ir/Ia, where Ir was the reactivation current density and Ia was the activation current density.
Figure 1: Double loop electrochemical potentiokinetic reactivation (DL-EPR) test
Atmospheric Corrosion Test
Atmospheric Corrosion Test is very important test to study the industrial environment effect on the materials. In this environment different type of industrial gases like sulphur dioxide, carbon dioxide, nitrogen oxides and other gases in the atmosphere vary from place to place depending on the dense of industries, and making the atmosphere very aggressive conditions for corrosion. Stainless steel coupons with a size 2×2 cm taken from cold rolled plates were exposed. The exposed coupons were hung in a hanging corrosion rack, which were facing at an angle 45o to the surface. Three sets of coupons were tested for the evaluation of atmospheric corrosion by weight loss method.
The corrosion rates were obtained by using the formula given below 15
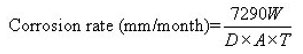
Where,
W=Weight loss (in gm)
A= Area of specimen (in cm2)
D= Density (in gm/cm3) for AISI 304SS, D=7.9 gm/ cm3
T=Time of exposure (in hours)
Pitting Corrosion Test
Pitting corrosion was investigated by conducting potentiodynamic polarization experiments at very slow scan rate 0.166mV/s in 0.01N NaCl and 0.05N HCl solution. The solutions were purged with pure nitrogen for an hour before sample immersion and were continued during the test. The critical pitting potential after passivity break down is indicated by the continuous increase in current in applied potential and has been used to delineate the susceptibility to pitting corrosion.
Results and Discussion
Hardness
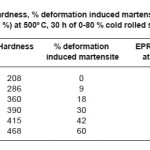
The measured hardness results indicated that it is a function of cold working reduction in thickness of the samples as shown in Table 1. Due to increasing cold working in thickness, corresponding decrease in grain size, enhanced residual stress and strain, increases the dislocation densities and vacancies. The hardness of the material has increased as a function of percentage cold deformation. It is due to the extent of induced martensite phase depending on the each cold deformation, presence in the materials.
Table 1: Hardness, % deformation induced martensite & EPR value (in %) at 500o C, 30 h of 0-80 % cold rolled samples
Deformation Induced Martensite
The induced martensite phase has been determined by XRD in cold rolled (Type 304) stainless steel and found to increase with increasing of cold rolled deformation. It is given in Table 1. It was observed that at a deformation of 80%, the martensite is a prominent phase in Type 304 SS. The extent of the martensitic phase in the material is related by increase in the hardness of the cold worked steels. The presence of martensite is potentially significant with respect to the sensitization process in two ways. The carbon and chromium activities are significantly different in this phase as compared to austenite. The chromium diffusivity would also be significantly different in a martensitic phase as compared to an austenitic phase. However, the deformation induced martensite is a metastable phase, which decreases with temperature.
Result of Double Loop Electrochemical Potentiokinetic Reactivation (DL-EPR) Test
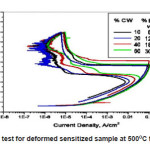
The stainless steels, in deformed as well as in non -deformed sensitized at 500oC for 30 hours, showed severity to intergranular corrosion. The result of electrochemical potentiokinetic reactivation (EPR) test showed that EPR value increased with increasing deformation of the stainless steel as shown in Table 1. The sensitization involves both the nucleation and growth of carbides at the grain boundaries depends on the state or the energy of the grain boundaries which provides the preferential sites for carbide nucleation and act as a favoured diffusion path for the growth of carbides. The grain-boundary precipitation of chromium-rich carbides and the formation of chromium-depleted regions were observed in the exposed samples in the temperature range of 500-700oC. The EPR values of the 0-60% deformed samples were calculated by degree of sensitization at 500oC for 30 hours as shown in Fig. 2. We have observed that the degree of sensitization increased with increasing deformation along with increased value of DIM. The severity of intergranular corrosion depends on the EPR value. The EPR value is a function of deformation. As the deformation increases the value of DIM increases.
Figure 2: EPR test for deformed sensitized sample at 500oC for 30 hours
Atmospheric Corrosion Test
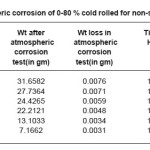
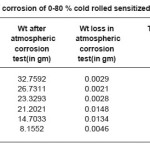
Although the data scatter is also present in the development of weight loss over time. Stainless steel coupons were exposed in open atmosphere for 17875 hours .The corrosion rate decreased with increased cold rolled deformation as shown in Table 2.The samples sensitized at 600o C for 1 hour showed decreased corrosion rate with increasing cold deformation in comparison to non deformed sensitized samples. The 40% and 60%cold rolled sensitized samples showed approximately equal corrosion rate of non deformed sensitized samples as shown in Table 3.
Table 2: Atmospheric corrosion of 0-80 % cold rolled for non-sensitized samples
Table 3: Atmospheric corrosion of 0-80 % cold rolled sensitized samples at 600°c/1hrs
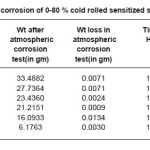
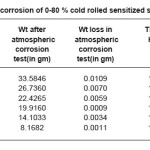
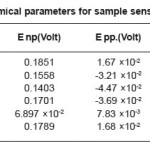
Samples sensitized at 600oc for 5 hours showed decreasing corrosion rate with increasing cold deformation in comparison to non deformed sensitized samples as shown in Table 4.We have observed adverse effect in the samples sensitized at 600oc for 30 hours as shown in Table 5.We have also observed that all samples sensitized at 600oc for 1 hour, 5 hours, 30 hours have lower corrosion rate in comparison to non sensitized as received materials.
Table 4: Atmospheric corrosion of 0-80 % cold rolled sensitized samples at 600°c/5hrs
Table 5: Atmospheric corrosion of 0-80 % cold rolled sensitized samples at 600°c/30hrs
The variation in the corrosion rate with cold rolling was non systematic for the received as well as sensitized samples. The samples were exposed in industrial environment on the roof of M.Tech hostel of National Institute of Foundry and Forge technology, Ranchi, India. They suffer due to the presence of atmospheric variables like climate condition, relative humidity, surface condition and polluted environment. The reason of corrosion rate enhancement in winter exposure in the presence of sulfur dioxide and chloride ion in polluted environment. These could break down protective surface films and imitate the corrosion .other major contaminants that promote atmosphere corrosion are nitrogen compounds, hydrogen sulfide and dust particle. The improved corrosion resistance of the sensitized samples was very interesting and was possibly due to redistribution of carbon or grain recrystallization.
Pitting Corrosion Test
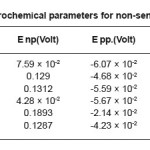
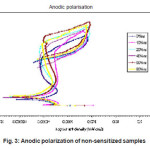
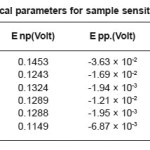
The stainless steels, in deformed as well as in non deformed state, showed resistance to pitting corrosion in 0.01(N) NaCl and 0.05(N) HCl solutions. However increases deformation changes the pitting resistance such that 60% deformed steel showed the heights pitting potential, Enb and resistance and 40% deformed steels showed the lowest pitting potential, Enb and resistance. We have also observed that corrosion potential increases with increasing deformation except 40%deformed state. The re-passivation potential increased very slightly with increasing deformation as shown in Table 6 and Fig 3.
Table 6: Electrochemical parameters for non-sensitized sample
Figure 3: Anodic polarization of non-sensitized samples
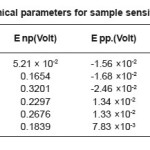
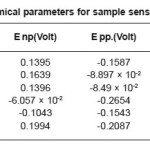
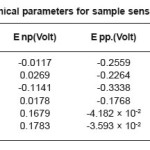
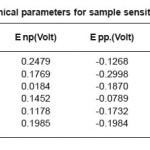
The change observed in pitting behavior could be due to the synergistic effects of residual stress deformation induced martensite, dislocation and vacancies as brought on by the deformation. After sensitization at 600o C for 1 hour, 5 hours, 30 hours. We observed pitting potential is greater than non-sensitized state except for the 60% cold rolled samples sensitized at 600o C for 5 hours and 30 hours. The 20% and 60% cold rolled samples sensitized at 600o C for 1 hour showed the highest pitting potential and corrosion resistance, the corrosion potential increased with decreasing time treatment at sensitization temperature while repassivation potential increased in comparison to non deformed samples but did not show a specific as shown in Table 6, Table7, Table8, and Table 9.
Table 7: Electrochemical parameters for sample sensitized at 600°C/1 hour
Table 8: Electrochemical parameters for sample sensitized at 600°C/5 hour
Table 9: Electrochemical parameters for sample sensitized at 600°C/30 hour
Samples sensitized at 700oC for 1 hour showed higher pitting potential for the 0 to 20% cold rolled samples and highest in the 80% cold rolled but lower in 40% and 60% deformed samples. The corrosion potential of samples sensitized at 700oC for 1 hour showed greater value than non sensitized samples while repassivation potential showed lower values. Samples sensitized at 700oC for 5 hours showed lower pitting potential than non-sensitized samples and 80% cold rolled sensitized samples showed slightly higher pitting potential, corrosion potential and repassivation potential than non-sensitized samples.40% cold rolled sensitized samples showed lower corrosion potential.
Samples sensitized at 700oC for 30 hours showed lower pitting potential for 20% and 60% cold rolled samples highest pitting potential for 0% and 80% cold rolled samples and higher values for 10% ,20% and 40% cold rolled samples .The corrosion potential of samples sensitized at 700oC for 30 hours showed higher values but lower repassivation potentials than the non sensitized samples as shown in Table 6 ,Table 10, Table 11 and Table 12. The change observed in pitting behavior in sensitized samples could be due to chromium carbide precipitate at the grain boundaries causing chromium impoverishment of the adjacent matrix. It is therefore expected that sensitized steel will be more susceptible to pitting corrosion. As the sensitized time and temperature were increased, leveling of chromium distribution occurred and rate of pitting decreased.
Table 10: Electrochemical parameters for sample sensitized at 700°C/1 hour
Table 11: Electrochemical parameters for sample sensitized at 700°C/5 hour
Table 12: Electrochemical parameters for sample sensitized at 700°C/30 hour
Conclusions
Deformation induced martensite increases with increasing reduction of thickness of the material through cold rolling.
A systematic correlation was observed between the Deformation Induced Martensite (DIM) phase and the Intergranular corrosion (IGC) susceptibility in our studies. The effect of DIM on degree of sensitization seems to be pronounced at 500OC when the steel retained significant amounts of DIM,
Atmospheric corrosion rate decreases with increasing thickness of the cold rolled deformation.
The atmospheric corrosion rate of sensitized samples at 600oC for 1hrs & 5hrs decreases in comparison to non sensitized samples. Exception were observed in only 60% sensitized cold rolled samples but in the case of fully sensitized samples at 600oC for 30 hrs were observed adverse effect.
Atmospheric corrosion in austenitic stainless steel had very less corrosion at exposure in their two years or more.
Samples sensitized at 700oC for 1 hour showed higher pitting potential for the 0 to 20% cold rolled samples and highest in the 80% cold rolled but lower in 40% and 60% deformed samples.
References
- A. Lebedev, V. Kosarchuk, Influence of phases transformations on the mechanical properties of austenitic stainless steels, International Journal of Plasticity 16: 749-767 (2000).
CrossRef
- Ravi Kumar, B, Mahato. B and Singh.Raghuvir. Influence of Cold-Worked Structure on Electrochemical Properties of Austenitic Stainless Steels Metallurgical and Materials Transactions A, 38: 2085-2094 (2007).
- J. Talonen, P. Nenonen, G. Pape, H. Hanninen, Effect of Strain Rate on the Strain Induced γ→α’, Martensite Transformation and Mechanical Properties of Austenitic Stainless Steels, Metallurgical and Materials Transactions 36A: 421-432 (2005).
CrossRef
- H. Shin, T. Ha, Y. Chang, and Kinetics of deformation induced martensitic transformation in a 304 stainless steel, Scripta Materialia 45: 823-829 (2001).
CrossRef
- Ravi Kumar B., et al., Metallurgical and Materials Transactions A, 36, pp. 3165-3174 (2005).
CrossRef
- Raghuvir Singh, I. Chattoraj, A. Kumar, B. Ravi Kumar and P. K. Dey. The effects of cold working on sensitization and intergranular corrosion behavior of AISI 304 stainless steel Metallurgical and Materials Transactions A, 34: 2441-2447 (2003).
CrossRef
- Dománková, Maria, Marek, Peter and Moravèík, Roman, The effect of cold work on the sensitization of austenitic stainless steels, Materials and Technology, 41: 131-134 (2007).
- Naeemi, A.H., and Albrecht, P (1984) Atmospheric Corrosion of Weathering Steel,Int.Cong.Metallic Corros.Toronto, Canada,1, 418-427.
- Money K. L, Metals Handbook Corrosion, Metals Park, Ohio, ASM International, 204 (1987).
- G-92-86 (Re-approved 1992) Standard practice for characterization of atmospheric test site Annual Book of ASTM Standards 03-02: 379-382 (1994). ASTM Philadelphia, A
- Khanna A.S, Atmospheric corrosion W.H. Ailor Ed, John, Wiley and sons Inc, New York NY, pp 461 (1982)
- Brown P.W and Masters L.W, Atmospheric Corrosion, Wiley, New York (1982).
- J. Baszkiewicz, M. Kaminski, Fundamentals of materials corrosion, Warsaw University of Technology Publishers, Warsaw. (in Polish) (1997).
- M. Ahlers, The Martensitic Transformation, Revista Materia 9: 169-183 (2004).
- C Garcýa, F Martýn, P.De Tiedra, J.A Heredero, and M.L Aparicio. Effect of prior cold work and sensitization heat treatment on chloride stress corrosion cracking in type 304 stainless steel, Corrosion Science 43:1519-1539 (2001).
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal