C.Yohannan Panicker1*, Hema Tresa Varghese2, B.Harikumar3 and Asha Chandran3
1Department of Physics, TKM College of Arts and Science, Kollam - 691 005, India.
2Department of Physics, Fatima Mata National College, Kollam - 691 001, India.
3Department of Chemistry, TKM College of Arts and Science, Kollam - 691 005, India.
DOI : http://dx.doi.org/10.13005/msri/080214
Article Publishing History
Article Received on : 01 Sep 2011
Article Accepted on : 12 Oct 2011
Article Published :
Plagiarism Check: No
Article Metrics
ABSTRACT:
The vibrational wavenumbers of methacrylonitrile were calculated using Gaussian03 software package at different levels of theory and the fundamental modes are assigned. The predicted infrared intensities and Raman activities are reported. The first hyperpolarizability is calculated and the title compound is an attractive object for futures studies of nonlinear optics. The calculated wave numbers (B3LYP) are in agreement with the experimental values.
KEYWORDS:
FT-IR; FT-Raman; DFT calculations; Hyperpolarizability
Copy the following to cite this article:
Panicker C. Y, Varghese H. T, Harikumar B, Chandran A. Vibrational Spectroscopic Investigations of Methacrylonitrile. Mat.Sci.Res.India;8(2)
|
Copy the following to cite this URL:
Panicker C. Y, Varghese H. T, Harikumar B, Chandran A. Vibrational Spectroscopic Investigations of Methacrylonitrile. Mat.Sci.Res.India;8(2). Available from: http://www.materialsciencejournal.org/?p=2656
|
Introduction
Methacrylonitrile is an aliphatic nitrile used extensively in the preparation of homo- and copolymers, elastomers, and plastics and as a chemical intermediate in the preparation of acids, amides esters and other nitriles. This aliphatic nitrile is also used as a replacement for acrylonitrile in the manufacture of an acrylonitrile/butadiene/ styrene-like polymer. Youcef et al.1 reported the study of ETFE based radiation grafted poly(styrene sulfonic acid-co-methacrylonitrile) proton conducted membranes with increased stability. Several authors reported on the use of styrene/ acrylonitrile,2,3 α-methylstyrene.styrene4 and p-methylstyrene/tert-butyl styrene5 as grafting monomers. Oh et al.6 reported an ab initio study of the molecular elimination reactions of methacrylonitrile. Extensitve studies have unveiled the aldoxime-nitrile pathway in microorganisms7,8 and the biological metabolism for nitrile compounds is increasingly noticed.9 In the present study, the IR, Raman and theoretical calculations of the wavenumbers of the title compound are reported.
Experimental
The FT-IR spectrum was recorded on a DR/Jasco FT-IR-6300 spectrometer in KBr pellets. The spectral resolution was 4 cm-1. The FT-Raman spectrum was obtained on a Bruker RFS 100/S, Germany. For excitation of the spectrum the emission of Nd:YAG laser was used, excitation wavelength 1064 nm, maximal power 150 mW, measurement on solid sample. The spectral resolution after apodization was 4 cm-1.
Computational Details
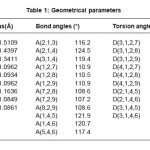
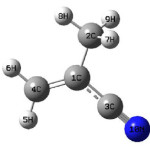
The vibrational wavenumbers were calculated using the Gaussian03 software package on a personal computer.10 The computations were performed at HF/6-31G(d) (6D, 7F) and B3LYP/6-31G(d) (6D, 7F) levels of theory to get the optimized geometry and vibrational wavenumbers of the normal modes of the title compound. DFT calculations were carried out with Becke’s three-parameter hybrid model using the Lee-Yang-Parr correlation functional (B3LYP) method. The wavenumber values computed contain known systematic errors11 and we therefore, have used the scaling factor values of 0.8929 and 0.9613 for HF and DFT basis sets. Parameters corresponding to optimized geometry of the title compound are given in Table 1. The absence of imaginary values of wavenumbers in the calculated vibrational spectrum confirms that the structure deduced corresponds to minimum energy.
Table 1: Geometrical parameters
Results and Discussion
The observed IR and Raman bands and calculated (scaled) wavenumbers and assignments are given in Table 2. In the spectra of methyl esters the overlap of the region in which both asymmetric stretching12 υasCH3 absorb with a weak to medium intensity (2985 ± 25 and 2970 ± 30 cm-1) is not large and regularly seen above 3000 cm-1. The computed wavenumbers (DFT) of modes corresponding to υasCH3 groups are 3023, 2992 cm-1. For the title compound, band at 2966, 2931 cm-1 in the IR spectrum and 3014, 2995 cm-1 in the Raman spectrum is assigned as asymmetrical methyl stretching modes. The symmetrical stretching mode υsCH3 is expected in the range 2920 ± 80 cm-1 in which all the three C-H bonds extend and contract in phase.12 The bands seen at 2937 (DFT), 2890 (IR) cm-1 and 2933 cm-1 (Raman) are assigned as these modes. Two bending vibrations can occur within a methyl group. With methyl esters the overlap of the regions in which methyl asymmetric deformations are active (1460 ± 25 and 1450 ± 15 cm-1) is quite strong, which leads to many coinciding wavenumbers.12 This is obvious, not only for the asymmetric deformations, but also for the symmetric deformations12 mostly displayed in the range 1380 ± 15 cm-1. The B3LYP calculations give 1463, 1447 and 1385 cm-1 as asymmetric and symmetric CH3 deformations for the title compound. Experimentally δasCH3 vibration is observed at 1452, 1428 cm-1 in the IR spectrum while δsCH3 vibrations are observed at 1381 cm-1 in the IR spectrum, 1404 cm-1 in the Raman spectrum. The methyl rocking wavenumbers are expected in the regions12 1100 ± 95 and 1080 ± 80 cm-1. The bands calculated at 1046, 1004 cm-1 are assigned as rocking modes of the methyl groups. The rocking mode is observed at 1018 cm-1 in the IR spectrum.
Scheme 1:
Table 2: Vibrational assignments
The vibrations of the CH2 group, the asymmetric stretch υasCH2, symmetric stretch υsCH2, scissoring vibration ωCH2 and wagging vibration υCH2 appear in the regions 3000 ± 50, 2965 ± 30, 1445 ± 55 and 1350 ± 85 cm-1, respectively.12,13 The CH2 stretching bands are observed at 3096, 2993 in IR spectrum and at 3114, 3041 cm-1 in the Raman spectrum. The scissoring and wagging vibrations were assigned at 1407 and 1247 cm-1 theoretically. The twisting mode τCH2 is assigned at 921 cm-1 theoretically as expected12.The rocking mode12 ρCH2 is expected in the range 895 ± 85 cm-1 and the band at 728 theoretically. The torsional mode of CH2 is seen the low wavenumber range12. The CH2 modes are reported at 2994, 2961 (stretching) and at 1143, 1168 cm-1(twisting) by Chandra et al..14 The methylene wagging exhibits absorption bands in the range 1360 ± 25 cm-1 and twisting mode in the range15-17 1290± 40 cm-1.
Nitrogen compounds featuring triple or cumulated double bonds, such as cyanides or nitriles (-C≡N) and cyanates –O-(C≡N), all provide a unique spectrum, typically with a single, normally intense absorption at 2280-2200 cm-1 (for cyano compounds) and 2285-1990 cm-1 (for cyanates, isocyanates and thiocyanates).12,18 In the present case the stretching mode υC≡N is observed at 2220 cm-1 in the IR spectrum and at 2230 cm-1 in the Raman spectrum. The calculated value for this mode is 2252 cm-1. Ca”N bond length19 is reported as 1.1361Å.
Nonlinear optics deals with the interaction of applied electromagnetic fields in various materials to generate new electromagnetic fields, altered in wavenumber, phase, or other physical properties.20 Organic molecules able to manipulate photonic signals efficiently are of importance in technologies such as optical communication, optical computing, and dynamic image processing.21,22 In this context, the dynamic first hyperpolarizability of the title compound is also calculated in the present study. The calculated first hyperpolarizability of the title compound is 0.48 × 10-30 esu and the title compound is an attractive object for future studies of nonlinear optical properties.
References
- Youcef, H.B., Gubler, L., Gursel, S.A., Henkensmeier, D., Wokaun, A., and Scherer, G.G., Electrochem. Commun. 11: 941 (2009).
CrossRef
- Becker, W., and Naake, G.S., Chem. Eng. Technol. 24: 1128 (2001).
CrossRef
- Becker, W., Bothe, M., and Naake, G.S., Angew. Makromol Chem. 273: 57(1999).
CrossRef
- Li, J., Muto, F., Miura, T., Oshima, A., Washio, M., Ikeda, S., Iida, Tabata, Y., Matsuura, C., and Katsumura, Y., Eur. Polym. J. 42: 1222 (2006).
CrossRef
- Chen, J., Asano, M., Yamaki, T., and Yoshida, M., J. Membr. Sci. 269: 194 (2006).
CrossRef
- Oh, C.Y., Park, T.J., and Kim, H.L., Bull. Korean Chem. Soc. 26: 1177 (2005).
CrossRef
- Asano, Y., J. Biotechnol. 94: 65 (2002).
CrossRef
- Kobayashi, K., Yoshioka, S., Kato, Y., Asano, Y., and Aono, Y., J. Biol. Chem. 280: 5486 (2005).
CrossRef
- Hashimoto, Y., Hosaka, H., Oinuma, K., Goda, M., Higashibara, H., and Kobayashi, M., J. Biol. Chem. 280: 8660 (2005).
CrossRef
- Frisch, M.J. et al. Gaussian 03, Revision C.02 Gaussian, Inc., Wallingford CT (2004).
- Foresman, J.B. in: Frisch, E. (Ed.), Exploring Chemistry with Electronic Structure Methods: A Guide to Using Gaussian Pittsburg, PA (1996).
- Roeges, N.P.G., A Guide to the Complete Interpretation of Infrared Spectra of Organic Structures, Wiley, New York (1994).
- Colthup, N.B., Daly, L.H., and Wiberly, S.E., Introduction to Infrared and Raman Spectroscopy, ed.3, Academic Press, Boston, MA, 1990.
- Chadra, S., Saleem, H., Sundaraganesan, N., and Sebastian, S., Indian J. Chem. 48A: 1219 (2009).
- Teixeira-Dias, J.C., De Carvalho, L.A.B., Da Costa, A.M.A., Lamperia, I.M.S., and Barbosa, E.F.G., Spectrochim. Acta 42A: 589 (1986).
CrossRef
- Ramis, G., and Busca, G., J. Mol. Struct. 193: 93 (1989).
CrossRef
- De Carvalho, L.A.E.B., Da Costa, A.M.N.A., Doarte, L.M., and Teixeria-Dias, J.J.C., Spectrochim. Acta 44A: 723 (1988).
- Coates, J., Encyclopedia for analytical chemistry, in: Meyers, R.A., (ed.) Interpretation of Infrared Spectrum, A Practical Approach, John Wiley and Sons Ltd., Chichester (2000).
- Varghese, H.T., Panicker, C.Y., Philip, D., and Pazdera, P., Spectrochim. Acta 67A: 1055 (2007).
CrossRef
- Shen, Y.R., The Principles of Nonlinear Optics, Wiley: New York, (1984).
- Kolinsky, P.V., Opt. Eng. 31, 1676 (1992).
CrossRef
- Eaton, D.F., Science, 253, 281 (1991).
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal