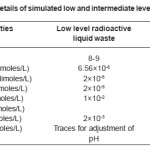
The composition of such waste generated in Indian reactors is as shown in table I (as supplied by Dr. S. D. Mishra, Director, Nuclear Recycle group, BARC, Trombay, Mumbai). Glasses were melted in three systems viz., a) Borosilicate system b) Phosphate system and c) Pure silicate system. Glass batches in the above three systems with predetermined compositions were synthesized in acetone medium. They are next dried and taken in a high quality alumina crucible and fired in muffle furnace. In melting operation, the temperature and time of melting are the principle factors to be monitored. The melting operation was done in a programmable muffle furnace with window and temperature controller. The temperature and time of melting was varied.
The effect of different modifier ions like Pb2+, Ba2+, Na+, Fe3+, Al3+, Y3+, Ca3+, Ce4+ and Sr2+ in each of the basic glass systems viz. borosilicate and phosphate networks on melting points and time of melting has been studied. X-ray diffraction study of the final products was made to confirm its amorphous nature. The pH of the leachate solution at ambient temperature was studied under varying time intervals.
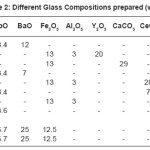
Ten glass forming systems (Table II), seven in Borosilicate system (BS1 to BS7), two in the Phosphate and one in pure Silicate system were studied. Quartz powder (AR grade, Oxford laboratory reagent, Mumbai) was used as the source of silicate and Borax (AR grade, RANKEM, New Delhi) in the borosilicate system, while both P2O5 (AR grade, E Merck) and H3PO4 (AR grade, E Merck) were tried in the phosphate composition. The glass with H3PO4 taken in the composition could not be melted even upto a temperature of 1100oC. It resulted only into a brown solid mass after leaching in the furnace. The respective temperature of melting and time of melting are shown in Table III. The simulated waste oxides like lead oxide (PbO, AR grade, Dipak laboratories, Kolkata), barium oxide (BaO, AR grade, BOROYNE, Mumbai), cerium oxide (CeO2, AR grade, Hi Media Laboratory, Mumbai), strontium oxide (SrO, AR grade, ALDRICH, USA) etc were chosen in the composition.
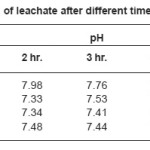
For the pH determination of the leachate solution, the bulk glass was powdered. This was done by grinding in a mortar. It was then allowed to pass through a sieve (size 330 and 420 mesh, B. S.). Accurately 1 g of the glass powder of definite size (0.30 – 0.42mm) was taken in a beaker with a fixed volume of distilled water of 40 c.c. It was stirred with a magnetic stirrer for a definite period of time of 2 mins. The pH of the liquid was determined by a pH meter (Systronics, Model no 335). Such measurement of pH was done at regular intervals of 1 hr, 2 hr, 3 hr, 4 hr and 5 hr respectively. The mixture was stirred after each 15 min with the help of the magnetic stirrer. Results of such pH study on selected glasses are shown in Table IV.
Results And Discussions
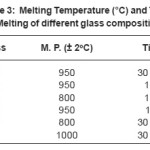
It was observed in the present work that for some initial melting operations (e.g. in BS1), there were heavy loss due to frothing. This resulted in loss of glass from the crucible. Increasing the former content (SiO2) in the composition (e.g. BS4) resulted in successful melts. In general it is found that the m. p. of glasses in the borosilicate system (BS1-BS7) is lowered in comparison to that in the phosphate system P1 (Table III). The bond dissociation energy of Si-O is 115 Kcal/mole, B-O is 125 Kcal /mole and P-O is 96 K Cal/mole. In borosilicate glass, the glass forms Si-O and B-O after dissociation. It is followed by recombination leading to liberation of higher amount of energy which is lowered in case of P-O bond energy.
The variation in melting points is due to mixed effect of different modifier ions, the exact trend being difficult to speculate. In the present work we could melt the glass composition at much lower temperature (800-950°C) with a soaking period of 30 min. to 1 hr. which were earlier reported to be melted not below 1000° C2. Considering the fact that the glass will be utilized to incorporate nuclear waste oxides with some of the fission fragments like oxides of Cs and Ru, which are volatile, lowering of glass processing temperature leads to the betterment of the utility of the glass melt.
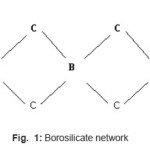
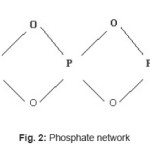
In general it is found that the melting point of glasses in borosilicate system (BS1-BS7) is lowered in comparison to that in the phosphate system (P-1) (Table III). Thus more energy and time is required to form the phosphate network. This is possibly due to the presence of simultaneous formers in borosilicate system, namely boron and silicon (Fig. 1) whereas in case of phosphate network,3 there is only one former atom (phosphorous, Fig. 2). The difference in bond strengths also contributes to this. In addition to these effects, the different modifier ions (added as oxides) can also lower the melting temperature of a particular composition of the phosphate system or borosilicate systems.4 Here the mechanism is that the modifier ion (Mn+) dissociates a Si-O (or B- O or P-O) bond of network and generates anionic O sites (O-) and gets attached to such sites ionically. Additionally the variation in m. p. among different compositions in the particular borosilicate system (BS1 to BS7) can be explained due to different types of modifier oxides being added.
As shown in Table IV there is a definite change in pH of the leachate solution between the phosphate system and the borosilicate system. The slightly increasing trend in the pH values in borosilicate system may be due to the mixed oxide network viz. [BO4] and [SiO4] in comparison to that in single oxide phosphate system.4-5 The observed pH values are in good agreement with the equilibrium pH values of glass system having composition close to present work. The range of pH obtained in a borosilicate glass with different modifier cation2 covers that of ours. It is seen from the table that there is a slight decreasing tendency as we go from 1 hr to 5 hr in steps of 1 hr. If we consider that the following dissociation is being operated in our aqueous system:
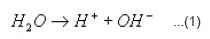
The extent of which (forward or reverse direction) is influenced by the different modifier ions released during the leaching of the glass sample.
The ionic radii (Å) of the different modifier ions in our case are as follows:
Pb2+ (1.33), Ba2+ (1.35), Na+ (0.95), Fe3+ (0.69), Al3+ (0.50), Y3+ (0.93), Ca2+ (0.99), Ce4+ (1.18) and Sr2+ (1.13).
The corresponding ionic potential (charge/radius) increases as Na+ (0.95) < Pb2+ (1.5)< Sr2+ (1.54) < Ba2+ (1.48) < Ca2+ (2.02) < Y3+ (3.23)< Ce4+ (3.39) < Fe3+ (4.69) < Al3+ (6.0)
Now greater the ionic potential of M2+ , the more it will attract OH– ions to form the corresponding hydroxides M(OH)n . The dissociation of H2O as above will be shifted to the forward direction releasing more number ofH+ in the medium. Thus the resulting solution will increasingly become acidic with lower value of pH.
This is reflected in our observed data clearly. Glass BS5 with Al2O3 (highest I. P.) shows lower value of pH than BS3 with no Al2O3 in the composition.
The decreasing trend in the pH value from 1 hr to 5 hr data can be explained in the light that with higher extent of leaching, more modifier cations are released into the solution increasing the ionic potential and therefore leading to lowering of pH values.
Table 1: Compositional details of simulated low and intermediate level radioactive liquid waste
Table 2: Different Glass Compositions prepared (wt %)
Table 3: Melting Temperature (°C) and Time of Melting of different glass compositions
Table 4: pH of leachate after different time intervals
Figure 1: Borosilicate network
Figure 2: Phosphate network
In the present work we could melt glass composition at much lower temperature (800- 950ºC) with a soaking period of 30 min. to 1 hr. This is on the lower side than the melting temperatures reported earlier (~1000º C). The lowering of temperature is possibly due to the presence of modifier ions. The difference in bond energies as well as number of glass formers (two in borosilicate compared to one in phosphate) might have contributed to difference in melting point in the two systems. The findings on pH of leachate solution have been explained in terms of ionic size, ionic radii and ionic potential of the modifier ions incorporated into the glass network. In order to reach a definite conclusion, more pH data of varying glass composition are necessary, the explanation of which will be obviously analytical.
Acknowledgements
Time to time discussions with Dr. J. Mukerji and Dr. A. S. Sanyal, retired Scientist, CGRI is duly acknowledged. The financial assistance rendered by DSA (Chemistry) and DSA (Physics), is also acknowledged.
References
- Plodinec M. J., Glass Technol., 41: 186 (2000).
- Mukerji J. and Sanyal A. S., Glass Technol., 45: 117 (2004).
- Nair M. K. T., “New Approaches in High Level Radioactive Waste Management”, Proc. Of Nuclear & Radio Chemistry Symp., (Eds.) V. Venugopal, B.S. Kumar and D. D. Sood, Andhra University., 41-44 (1992).
- Raman S. V., J. mater. Res., 13: 8 (1998).
CrossRef
- Kamizono I., Hayakawa S. and Muraoka S.,J. Materials Science Letters, 10: 423 (1993).
CrossRef
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal