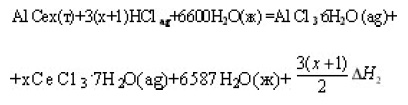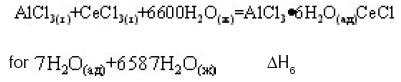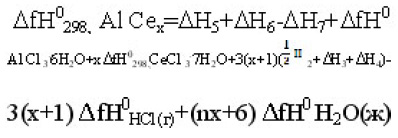Preparation of Physical and Chemical and Thermodynamic Properties of Aluminum Alloys-Cerium
M. Razazi1, R. N. Amini1, B. B. Eshov2 and A. B. Badalov3
¹Department of Material Science and Engineering, Isalmic Azad University, Majlesi Branch, Isfahan, Iran.
²Chemistry Institute, Tajikistan Academy of Sciences, Dushanbe, Tajikistan
³Department of Chemical Technology and metallurgy,Tajik Technical University named after academician M.S.Osimi, Dushanbe, Tajikistan.
DOI : http://dx.doi.org/10.13005/msri/090101
Article Publishing History
Article Received on : 20 March 2012
Article Accepted on : 07 May 2012
Article Published :
Plagiarism Check: Yes
Article Metrics
ABSTRACT:
Chemical compositions, microstructure and hardness of alloys and Al - Ce intermetallic compounds have been studied. The method of calorimetry solution determined the enthalpy of dissolution. Enthalpy of formation of intermetallic compounds In thermo chemical cycle has been calculated. It was revealed the regularity of their changes depending on the composition. Melting temperature of intermetallic compounds of the system has been specified by Semi empirical method. It has defined the specified set pattern in the changes on melting temperature and enthalpy of formation of IM on the concentration of Maximal stability in the composition of Al2Se.
KEYWORDS:
Alloy; intermetallic; Al; lanthanides; the heat of solution; enthalpy of formation
Copy the following to cite this article:
Razazi M, Amini R. N, Eshov B. B, Badalov A. B. Preparation of Physical and Chemical and Thermodynamic Properties of Aluminum Alloys-Cerium. Mat.Sci.Res.India;9(1)
|
Copy the following to cite this URL:
Razazi M, Amini R. N, Eshov B. B, Badalov A. B. Preparation of Physical and Chemical and Thermodynamic Properties of Aluminum Alloys-Cerium. Mat.Sci.Res.India;9(1). Available from: http://www.materialsciencejournal.org/?p=1156
|
Introduction
Study of Fundamental physical – chemical, mechanical, thermal and thermodynamic properties and the resulting information is the reliable scientific basis for a successful search and development of new compounds and materials with predetermined properties. Such studies for metallic systems based on aluminum and lanthanides are particular relevance. Widespread use of compounds of these metals, due to make special, sometimes unique, characteristics of various branches of modern engineering and technology1-3. Aluminum is the founder of III A and III B subgroups of the Periodic System of chemical elements. It is in the group which is the most clear manifest all kinds of analogies4.
On the other hand, the great interest in the chemistry of the lanthanides due to use much raw material of lanthanides, advance in chemical technology division and getting them to a high degree of purity.
In the scientific aspect of the features of the electronic structure and expression of polyvalence in similar lanthanide compounds and metallic systems contribute to the expansion of knowledge in the theory of chemical bonding. The obtained data will join to the bank of thermodynamic quantity with new data, valuable information regarding the boundary conditions of metallic systems, the structure of a particular material of phase composition and equilibrium phase diagrams5,6. Currently the phase diagram of aluminum constructs almost with all the rare-earth metals6-9. Rare-earth metals slightly are soluble in aluminum at room temperature. Increasing temperature due to increase in solubility. A diagram of the system is characterized by the formation of the eutectic of the aluminum and a number of chemical compounds8.
Experimental
In the continuation of the works10,11, we have studied the properties of (IM) intermetallic Al-Ce system and alloys According to the done works6-9, in the form of the intermetallic system of compounds:
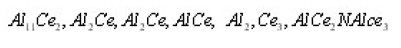
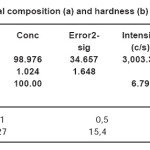
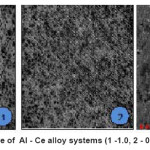
Aluminum-based alloys and IM were obtained in a vacuum resistance furnace under an inert gas at an excess pressure of 0.15 MPa in the alumina crucibles. The initial charge materials used aluminum (99.99 wt.%), Cerium (99.9%). The concentration of cerium in the alloys ranged from 0.05 to 1.0 wt.%. Investigation of the composition (see Table 1) and structure (Fig. 1) alloys was carried out with an electron microscope SEM 2100 (Korea). Selective analysis of the quantitative composition showed that the discrepancy did not exceed 2% of the calculated values.
Determination of hardness of alloy according to Brinell method was carried out by standard methods for hardness by apparatus COUPAL. Structure of alloys consists of a solid solution of á-Al + eut. (á-Al+Al11Ce3) As the concentration of cerium fraction of the inclusion of this IM and the hardness of alloys increases. Slight increase in hardness due to the purity of aluminum alloys.
The values are applied to search for methods to assess and predict the thermal properties of alloys of aluminum – lanthanides. We use the call of semi-empirical method to predict the melting temperature of Al – Ce intermetallic systems [12], which takes into account the effect of 4 f – electrons (Nf), the contribution of the spin (S) – and
orbital (L) – the moments of the ions of the lanthanides on the property under the studying of IM ( A). The results of calculations have been made based on the correlation equation which are shown in table 2:
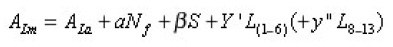
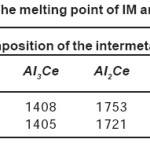
Table 2 shows that the melting point of IM increased with increasing concentration of cerium in the composition of Al2Ce and decreases at higher cerium contents. The enthalpy of dissolution of intermetallic compounds and Al – Ce alloys has been determined by calorimetrical method with isothermal shell. The calorimeter unit includes a thermostat, sealed calorimetric cell, Bridge R-329 (accuracy class 0.05%), EZ-2 recorder with a sensitivity of 10 -10 ampere, the unit of calibration and precision voltage stabilizers. Thermometerical and thermal sensitivity of the calorimeter were respectively 10-4 Ê and ±0,08 J.
The calorimeter is calibrated by setting the heat of dissolution of potassium chloride. The quantity of heat equal to the KCl solution

The optimal solvent to dissolve alloys and intermetallic compounds was 0.5 M solution of hydrochloric acid. Samples of the studied substances were very small (less than 2’”10-3 mol) compared to the amount of solvent (250 ml). The degree of dilution of the solution of sample after dilution was 1:1100. The heat of dilution of solutions of hydrochloric acid is taken into account for the reference [13]. Experimental values have obtained with the averaged values of three values (in a good match) and five experiments.
The reaction has been measured the heat of solution of aluminum metal in 0,5M hydrochloric acid. The value of the enthalpy of formation of hydrated aluminum ions determined from thermo chemical cycle
2Al(τ)+6HCl(Ψ)+6600H2O(æ)=2Al3+(OH2)6+6Clag +3H2+6588H2 ΔH1
and is equal to 3(½Δ H2+Δ H3+Δ H4)= – 1317,75. J [13].
The value set from the enthalpy of formation of hydrated ions Al3+ ag, equal to 529.6 kJ.
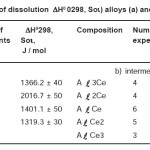
It is well coincides with the references [13]. The results of the study provided a process of dissolution of alloys and intermetallic compounds of Al-Ce which are shown in table 3.
Table 3 shows studied changes in the narrow range in the concentration of cerium in the alloys. There is a noticeable increase in the value of the enthalpy of dissolution for the content of 0.1 wt.% Cerium. With further increase of cerium content in the alloy, enthalpy of dissolution of alloys decreases to its previous value and becomes almost a straight character within the experimental error. Such a change in the enthalpy of solution of alloys, possibly due to the solubility limit of cerium in aluminum.
Dependence of enthalpy of dissolution of studied composition shows the concentration range divides into two zones with a minimum value for the composition of A–!2Ce. The obtained values of enthalpy of dissolution of intermetallic compounds allow the calculation of the enthalpy of formation in accordance with our thermochemical cycles. The process of dissolution of IM systems, A –!-Ce in the solution of hydrochloric acid is expressed by the equation:
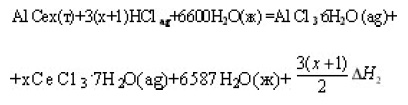
Where x is the index of cerium in the IM. The processes are expressed by the equations:
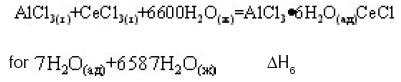
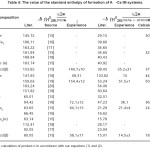
Using the values of the enthalpy of dissolution of IM (Table 3) and the reference values of the standard enthalpy of formation of other components of the system [13,14] due to calculate the enthalpy of formation of Al-Ce studied system, which are listed in table 4.
Table 1: Chemical composition (a) and hardness (b) of Al-Ce alloys
Table 2: The melting point of IM and Al – Ce
Table 3: The enthalpy of dissolution ΔH0 0298, Soι) alloys (a) and (b) Al-Ce IM system
Calculation is made for the thermochemical cycle
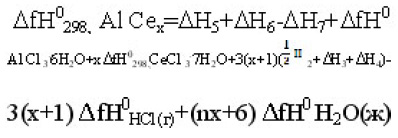
Table 4: The value of the standard enthalpy of formation of A l-Ce IM systems.
Results and Discussion
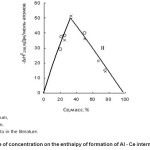
Summarizing the literature and experimental values of enthalpy of formation of IM systems and Al-Ce system analysis carried out by dependence of concentration within the system (Fig. 2). Area of concentration dependence on the enthalpy of formation of IM is divided into two zones with a maximum value of the enthalpy of formation at a concentration of IM (XAl )= 66.7% wt., are corresponded to the composition of Al2Ce. This picture differs from the literature of the lack of third
in the transition zone6.
Analysis and processing of available information on the magnitude of the enthalpy of formation of Al-Ce IM systems allowed to derive the expressing equations of the dependence of the thermodynamic characteristics MI on the concentration. Concentration on the enthalpy of formation of IM in the area with a high content of aluminum (zone I) is expressed by the following equations of a straight line
Δf H 0 298, IM = 1.517 X (1)
where X is the content of Ce,% wt.
Figure 1: The microstructure of Al – Ce alloy systems (1 -1.0, 2 – 0,5; 3 – 0,1 Wt.% Ce) (x100)
Figure 2: Dependence of concentration on the enthalpy of formation of Al – Ce intermetallic compounds
The zone II is corresponded to a low content of aluminum, this dependence is expressed by the equation
ΔH f 0298, IM = – 0.736 x + 73.58 …(2)
Conclusion
Increasing concentration of cerium in the Al-Ce IM system was observed increasing in the melting temperature of Al-Ce. At higher concentrations of cerium lowering the melting point of the system was observed; Applied experimental method of calculating the melting temperature of IM aluminumcerium systems can be successfully used to assess the physico-chemical and thermodynamic properties of similar systems with lanthanides; In the investigated system, maximum thermal and thermodynamic stability of the aluminum-cerium intermetallic compound has Al2Ce, which has the smallest value of the enthalpy of dissolution in acid and the maximum value of the enthalpy of formation. The data obtained allow to targeted the synthesis of intermetallic materials and the specific composition with predetermined properties.
References
- V. Serebrennikov chemistry of rare earth elements. B2’s so-Vol.1-Tomsk: Tomsk State University in 362 (1959).
- Zelikman AN Meyerson GN Metallurgy of rare metals. M. Metallurgiya. 608 (1973).
- K. Taylor, Intermetallic compounds of rareearth metals. M. Mir., 224 (1974).
- YA Ugai General and Inorganic Chemistry. M.: Higher. School. In. 527 (2004).
- O. Kubaschewski, SB Olkkok Metallurgical thermochemistry. Moscow: Metallurgy. In 280 (1982).
- VA Lebedev, Kober, VI, A Yamschikov. F. Thermochemistry of alloys of rare earth and actinide metals. Chelyabinsk: Metallurgiya., 366s (1989).
- LF Mondolfo Structure and properties of aluminum alloys. M. Metallurgy. In 1979. – 639 p.
- The phase diagram of binary metal systems: D44 Reference: In 3t. :/ Under the general editorship. H. P. Lyakisheva. M.: Machinery . 992 (1996).
- Binary alloy phase diagrams. V.1.250 P. American Society for metals. Metals Park Ohio. In 1986. Editor in Chief Ma ssalski T. B.
- Badalov A. B., Mirzoev Sh. And., Ganiev I. H. , Eshowe B. B. / / Dokl. Republic Tajikistan. In 2005. M. XLVIII. ¹ 9.10. – With the .86-90.
- Eshov B. B., Mirzoev Sh. I., Nuriddinov B.Sh., Badalov AB / / Abstracts of XVII Inter.conf. on Chemical Thermodynamics in Russia, Kazan, 29.06-03.07, 2: 115 (2009).
- Poluektov NS, SB Meshkov, Korovin YV, Oksinenko II / / Dokl. USSR Academy of Sciences. In t.266. Number 5. – S.1157-1159 (1982).
- A. Volkov, IM Zharsky Large Chemical Directory. Minsk: Sovrem.shk. In 608 (2005).
- Panyushkin VT Afanas’ev Yu, Han H., Granovsky, AD, OA Osipov lanthanides. Simple and complex compounds. Russia. Rostov-on-Don: Izd Rostov.univ. In 296 (1980).
- [May 1] Gao MC, Unlu N., Shitlet GL, Mihalovich M. / / Metallurgical and Materials Trans. A., 36: 3262-3279 (2005).
- Sommer F., Keita M. / / J.Less-Comm. Met., , 136: 95-99 (1987).
- Thermal Constants substances. Guide (under Ed. Acad. VP Glushko). -M.: AN SSSR, VINITI, ICT, 8: ch.1.-570s (1978).
- Borzont G., Cacciamane G., Ferro R. / / Metall.Trans.A, 22A: 2119-2123 (1991).
- Colinet G., Pastuel A., Buschow KH / /J.Chem. Therodyn., 17: 1133-1139 (1985).
CrossRef
- Hultgren R., Desai PD, Hawrins DT at. all. / / Metalls Park, Ohio: ASM, 1433p (1973).

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal