Shahriar Ghammamy* and Sajjad Sedaghat
1Department of Chemistry, Faculty of Science, Islamic Azad University, Malard Branch, Malard (Iran).
DOI : http://dx.doi.org/10.13005/msri/090106
Article Publishing History
Article Received on : 20 March 2011
Article Accepted on : 07 May 2011
Article Published :
Plagiarism Check: Yes
Article Metrics
ABSTRACT:
We describe the synthesis and characterization of a new Co(III) complex of the Schiff base ligand (N1 E, N2E) - N1-((1H- pyrrol-2-yl) methylene) - N2-(1- phenylethylidene) ethane- 1,2- diamineabbreviated as MPEAwas synthesized and characterized.Co (III) Metal complex of this Schiff base ligand prepared by reaction of CoCl2.6H2O with MPEA. Characterization of the ligand was made by microanalyses, FT-IR, 1HNMR and 13CNMR and its complex was made by FT-IR.
KEYWORDS:
words:(N1 E; N2E) - N1-((1H- pyrrol-2-yl) methylene) - N2-(1- phenylethylidene) ethane- 1; 2- diamine; Co (III) complex; synthesis; 1HNMR; 13CNMR
Copy the following to cite this article:
Ghammamy S, Sedaghat S. Synthesis and Characterization of Co (III) Complex of the New Chiral Schiff Base. Mat.Sci.Res.India;9(1)
|
Copy the following to cite this URL:
Ghammamy S, Sedaghat S. Synthesis and Characterization of Co (III) Complex of the New Chiral Schiff Base. Mat.Sci.Res.India;9(1). Available from: http://www.materialsciencejournal.org/?p=1171
|
Introduction
Schiff bases have often been used as chelating ligands in the field of coordination chemistry and their metal complexes are of great interest for many years. It is well known that N and S atoms play a key role in the coordination of metals at the active sites of numerous metallobio molecules1. Schiff base metal complexes have been widely studied because they have industrial, antifungal, antibacterial, anticancer and herbicidal applications2-3. They serve as models for biologically important species and find applications in biomimetic catalytic reactions. Chelating ligands containing N, S and O donor atoms show broad biological activity and are of special interest because of the variety of ways in which they are bonded to metal ions. It is known that the existence of metal ions bonded to biologically active compounds may enhance their activities4-6. The variety of possible Schiff base metal complexes with wide choice of ligands, and coordination environments, has prompted us to undertake research in this area7. Zinc can function as active site of hydrolytic enzymes, where it is ligated by hard donors (N or O). It has long been recognized as an important co-factor in biological molecules, either as a structural template in protein folding or as a Lewis acid catalyst that can readily adopt the coordination numbers 4, 5, or 6 8-10. The catalytic role of Zn comprises Lewis acid activation of substrate, generation of a reactive nucleophile (Zn- OH) and stabilization of the leaving group. As a part of our continuing work on dissymmetric tetradentate Schiff base complexes containing N, S and O donor atoms [11] and in light of the importance of Cd and Zn ion metals, recently, We now report the synthesis and characterization of Co (III) complex of the Schiff base ligand (N1 E, N2E) – N1-((1H- pyrrol-2-yl) methylene) – N2-(1- phenylethylidene) ethane- 1,2- diamine.
Experimental
Material and Method
All reagents were supplied by Merck and were used without further purification. Melting point was determined in an Electrothermal 9200. The FT IR spectra were recorded in the range 400–4000 cm-1by KBr disk using a Bruker Tensor 27 M 420 FTIR spectrophotometer.The NMR spectra were recorded by Bruker250 MHz Avance andBruker300 MHz Avance.
Synthesis of the (E)- N1-(1- phenylethylidene) ethane- 1,2-diamine Ligand
For synthesis of the (E)- N1-(1- phenylethylidene) ethane- 1,2-diamine ligand, to a magnetically stirred of acetophenone(3.70 g, 30mmol) was added ethylene diamine(1.85g, 30mmol) via a syringe and in 60-900C for 24h was reflexed.
Synthesis of the MPEALigand
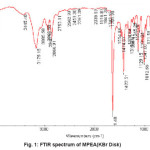
(E)- N1-(1- phenylethylidene) ethane- 1,2- diamine (1.36g, 8.4mmol) and 2-pyrrol carbaldehyde (0.8g, 8.4mmol) were dissolved at room temperature. The mixture was stirred and heated for 4 h to give a clear solution. After cooling to room temperature, the resulting white precipitate was filtered. Mp1780C Yield:80%. Anal. Calc. for C15H17N3: C, 75.31; H, 7.11; N, 17.57%. Found: C, 75.55; H, 7.28; N,17.85%. FTIR (KBr pellet, cm-1): 3175.1(w, NH), 2866.9 (w,=CH); 641 (m, C=N), 1012.6 (w, CH), 1HNMR (ä ppm CDCl3, 300MHz): 2.6 (CH3), 3-4 (-CH2-CH2-), 6-7.5 (phenyl group and hetro atomic group), 8-9 (=CH-), 9-10 (hexchang (- NH)).13CNMR (ä ppm CDCl3, 300MHz): 20-40 (- CH3), 50-60 (-CH2-CH2-), 100-140 (phenyl group and hetro atomic group), 140-160 (=CH), 160-180 (PH-C=N) (Figure 1, 2, 3).
Synthesis of the Co (MPEA)
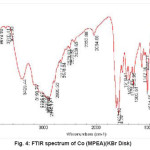
MPEA(1.2g, 3mmol) and CoCl2.6H2O (0.7g, 3mmol) were dissolved in CH3CN. The mixture was stirred for 3 h to give a clear solution. The resulting dark green mixture was filtered. Yield:83%. Anal. Calc. for C15H17N3Co: C, 60.41; H, 5.70; N, 14.09%. Found: C, 60.75; H, 5.95; N,14.20%. FTIR (KBr pellet, cm-1): 3133.7(w, NH), 2805.52 (w,=CH); 1625.92 (w, C=N), 1034.5 (w, CH). (Figure4).
Results and Discussion
Schiff bases are potentially capable of forming stablecomplexes with metal ions. Schiff bases form a significant class of compounds in medicinal and pharmaceutical chemistry with several biological applications that include antibacterial [1-6]’’, antifungaland antitumor activity.Schiff base complexes play a vital role in
Figure 1: FTIR spectrum of MPEA(KBr Disk)
Figure 2
Figure 3: 13C-NMRspectrum of MPEA (in CDCl3)
Figure 4: FTIR spectrum of Co (MPEA)(KBr Disk)
designing metal complexes related to synthetic and natural oxygen carriers Cobalt (III) saltreacts with Schiff base ligand in 1:1(L/M) molar ratio in solvent to afford complex. The ligandand complex are stable at room temperature. In this paper, a direct, simple and one step method has been used to synthesize these compounds. The advantages of the method are; that there is no side product, the reaction is quite fast, there are mild conditions, and the accompanied color change that provides visual
means for ascertaining the progress of the reaction. In summary, the synthesis and characterization of complex have been described. A complex of CO (III) was synthesized simply. Co (MPEA)was prepared by the reaction of MPEA and CoCl2.6H2O. In this study we have reported the synthesis of a new diaminederivative and it, s Co (III) complex. The structural characterizations of synthesized compounds were made by using the elemental analysis, IR, 1HNMR and 13CNMR techniques.
These MPEA ligand and Co (III) compound were obtained in relatively high yield, 80 and 83% respectively.The infrared spectrum of the complex and ligand was obtained.The IR spectra of the Schiff base show characteristic bands due to ν(NH), ν(=CH), ν(C=N) and ν(CH) in the region 3175.1 cm-1, 2866.9cm-1, 641cm-1and 1012.6cm-1, respectively. In the case of Co (III) complex we observed characteristic bands due toí(NH), ν(=CH), ν(C=N) and ν(CH)in the region 3133.70 cm-1, 2805.52cm-1, 1625.92cm-1and 1034.5cm-1 respectively.The 1H-NMR spectra of MPEA compound displays a signal at 206 ppm which is assigned to CH3, a signal at 3-4 ppm which were assigned to –CH2-CH2-, signals at 6-7.5 ppm which are assigned phenyl group and hetro atomic groupand one signal at 8-9 ppm which is assigned
to =CH.
Acknowledgement
We gratefully acknowledge the financial support from the Research Council of Takestan Islamic Azad University and many technical supports that provided by Tarbiat Modarres University.
References
- Inorg Met-Org Chem., 29: 105 (1999).
- Chandra S.,SangeetikaJ.,Indian Chem., 81: 203 (2004).
- YildizM.,DulgerB.,DulgerS.Y., Koyuncu B.M.,Yapici., J. Indian Chem. Soc., 81:7(2004).
- SinghK.,BarwaM.S.,Tyagi P,Eur. J. Med. Chem.,42: 394 (2007).
CrossRef
- Ferrari M.B.,CapacchiS.,Pelosi G.,ReffoG.,TarasconiP.,Albertini R.,Pinelli S.,Lunghi P., Inorg. Chim. Acta., 286: 134 (1999).
CrossRef
- Canpolat E.,KayaM., Chem.J. Coord.,57: 1217 (2004).
CrossRef
- Cozzi, P.G., Chem. Soc. Rev.,33: 410 (2004).
CrossRef
- Majumder A.,Rosair G.M., Mallick A., Chattopadhyay N.,MitraS., Polyhedron., 25: 1753 (2006).
CrossRef
- Sun,X.X., Qi,C.M., Ma, S.L., Huang,H.B., Zhu,W.X., Liu, Y.C, Inorg. Chem. Commu., 9: 911 (2006).
CrossRef
- Saghatforoush, L.A., Hossaini Sadr, M., Lewis, W., Wikaira, J., Robinson, W.T. and Ng, S.W., ActaCrystallographica, Section E: Structure Reports Online.,1259: 60 (2004).
- LipscombW.N.,Strater, N., Chem. Rev., 96: 2375 (1996).
CrossRef
- ValleeB.L.,AuldD.S., Acc. Chem. Res., 26: 543 (1993).
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal