Synthesis, Spectroscopic Properties of Nickel (II) Complex of the New Bis Amide Ligand N, N'- Alkanelid Bis Amide [Ni(NABA)]+
Shahriar Ghammamy
Department of Chemistry, Faculty of Science, Islamic Azad University, Malard Branch, Malard, Iran.
Article Publishing History
Article Received on : 03 Mar 2012
Article Accepted on : 15 Apr 2012
Article Published :
Plagiarism Check: No
Article Metrics
ABSTRACT:
We describe the synthesis and characterization of a new Nickel (II) complex of the bis amide ligand N, N’- alkanelid bis amide that abbreviated as NABA was synthesized and characterized. Ni (II) Metal complex of this bis amide ligand was prepared by reaction of chloride salt of Ni (II) with NABA. Characterization of the ligand was made by microanalyses, FT-IR,1HNMR and its complex was made by microanalyses, FT-IR and UV–Visible spectroscopy.
KEYWORDS:
N; N’- alkanelid bis amide; Ni (II) complex; Synthesis; Spectroscopic properties
Copy the following to cite this article:
Ghammamy S. Synthesis, Spectroscopic Properties of Nickel (II) Complex of the new Bis amide Ligand N, N?- alkanelid bis amide [Ni(NABA)]+. Mat.Sci.Res.India;9(1)
|
Copy the following to cite this URL:
Ghammamy S. Synthesis, Spectroscopic Properties of Nickel (II) Complex of the new Bis amide Ligand N, N?- alkanelid bis amide [Ni(NABA)]+. Mat.Sci.Res.India;9(1). Available from: http://www.materialsciencejournal.org/?p=1208
|
Introduction
Bis amides have often been used as chelating ligands in the field of coordination chemistry and their metal complexes are of great interest for many years. It is well known that N atom plays a key role in the coordination of metals at the active sites of numerous metallobio molecules.1 Bis amides metal complexes have been widely studied because they have industrial, antifungal, antibacterial, anticancer and herbicidal applications.2-3 They serve as models for biologically important species and find applications in biomimetic catalytic reactions. Chelating ligands containing N, S and O donor atoms show broad biological activity and are of special interest because of the variety of ways in which they are bonded to metal ions. It is known that the existence of metal ions bonded to biologically active compounds may enhance their activities.4-6 The variety of possible bis amides metal complexes with wide choice of ligands, and coordination environments, has prompted us to undertake research in this area.7 Nickel can function as active site of hydrolytic enzymes, where it is ligated by hard donors (N or O). It has long been recognized as an important co-factor in biological molecules, either as a structural template in protein folding or as a Lewis acid catalyst that can readily adopt the coordination numbers 4, 5, or 6.8-10 The catalytic role of Ni comprises Lewis acid activation of substrate, generation of a reactive nucleophile and stabilization of the leaving group. As a part of our continuing work on dissymmetric tetradentate bis amides complexes containing N, S and O donor atoms11 and in light of the importance of Ni ion metal, recently, there has been a considerable interest in the chemistry of bis amides compounds because of their potential pharmacological applications. Several reports on synthesis of metal complexes of bis amide by the use of organic solvents under reflux and their biological properties have already been published. More so, these methods suffer several drawbacks such as long reaction time, an excess of organic solvent, lower products yield and harsh refluxing conditions. We now report the synthesis and characterization of Ni (II) complex of the N, N’- alkanelid bis amide ligand.
Experimental
Material And Method
All reagents were supplied by Merck and were used without further purification. Melting point was determined in an Electrothermal 9200. The FT- IR spectra were recorded in the range 400–4000 cm-1 by KBr disk using a Bruker Tensor 27 M 420 FT-IR spectrophotometer. The UV–Vis spectra in CH3CN were recorded with a WPA bio Wave S2 100 spectrophotometer. Elemental CHN analyses were performed using a Heraeus CHN-O-RAPID elemental analyzer.
Synthesis Of The NABA Ligand
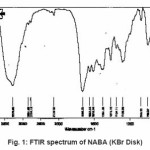
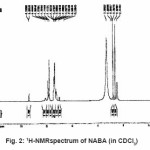
For synthesis of the NABA ligand, to a magnetically stirred of acetamide (1.13 g, 10mmol) in CH2Cl2 (10 ml) and hydrochloride acid (10ml) was added 4-methylbenzaldehyde (0.53g, 5mmol) via a syringe and heated for 4 h. After cooling to room temperature, the resulting white precipitate was filtered, washed with n-hexane (20 ml) and dried. M.P.: 235-237º C Yield: 89%. Anal. Calc. for C11H14N2O2: C, 64.07; H, 6.79; N, 13.59%. Found: C,64.18; H, 6.95; N, 13.83%. IR (KBr Disk, cm-1): 3279(w, N-H), 3111(w, C=O); 1381 (w, C=N); 690(s,C-H). (Figure1, 2, Table 1).
Figure 1: FTIR spectrum of NABA (KBr Disk)
Figure 2: 1H-NMRspectrum of NABA (in CDCl3)
Table 1: FTIR spectral data (cm-1) of NABA
Preparation Of [Ni(Naba)]+ Complex
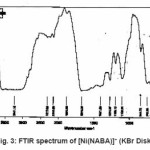
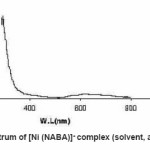
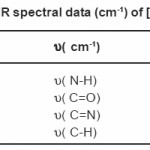
NABA (0.5g, 1mmol) and NiCl2 (0.3g, 0.5 mmol) were dissolved at room temperature. The mixture was stirred and heated for 4 h to give a clear solution. After cooling to room temperature, the resulting green precipitate was filtered, washed with n-hexane (20 ml) and dried. M.P.: 112-1140C Yield: 91%. Anal. Calc. for C11H14N2Cl2NiO2: : C, 49.81;H, 5.28; N, 10.56%. Found: C, 49.95; H, 5.45; N,10.63%. FTIR (KBr pellet, cm-1): 3241(N-H), 1674 (w, C=O); 1274 (w, C=N), 590 (s, C-H), UV–Vis, λmax (nm)/ε (M-1cm-1); 599 (67), 386 (77), 272 (136).(Figure3, 4, Table 2). This compound soluble in ethanol, methanol, acetonitrile, DMSO, acetone and chloroform solvents and in toluene and petroleum ether is less soluble and in water, ether and hexane is insoluble.
Figure 3: FTIR spectrum of [Ni(NABA)]+ (KBr Disk)
Figure 4: UV / Vis spectrum of [Ni (NABA)]+ complex (solvent, acetonitrile, 5×10-4 M)
Table 2: FTIR spectral data (cm-1) of [Ni (NABA)]+
Results And Discussion
The nickel ion in nickel (II)-complexes exists in the coordination number of 4, 5 and 6 and octahedral, trigonal- bipyramidal, quadratic- pyramidal and tetrahedral complexes are paramagnetic and have in the majority of cases a green or blue color. The quadratic-planar nickel complexes are diamagnetic and mostly have a yellow, red or brown color.
The synthesis of nickel (II)-complexes passes over ligand substitution reactions where one or several ligands are replaced by other ones.These reactions are equilibrium reactions.
Essential for the building of a new complex is the complex building constant (stability constant). In all the following reactions a more basic ligand is removed by a lower basic one or a less dentate one by a more dentate one.
In this study we have reported the synthesis of a new bis amide derivative and it, s Ni(II) complex. The structural characterizations of synthesized compounds were made by using the elemental analysis, IR and UV spectral techniques. From the spectroscopic characterization, it is concluded that ligands acts as a neutral bidentate through the azomethine nitrogen atom and carbonyl groups.
Synthesis and Stability
Ni (II) salt reacts with Bis amide ligand in 1:1(L/M) molar ratio in solvent to afford complex. The ligand and its complex are stable at room temperature. These NABA ligand and (N, N’- alkanelid bis amide nickel (II) compound were obtained in relatively high yield, 89 and 91% respectively. These title compounds are soluble in solvents such as methanol, ethanol, acetonitrile, DMSO, acetone and chloroform, less soluble in in toluene and petroleum ether and in soluble in water, ether and hexane too. The strong band at 3279 is assigned to the N-H, 690 cm-1 is assigned to the C- H, 3111 cm-1 is assigned to the -C=O- and 1381 is assigned to -C=N- stretching vibrations compound in NABA ligand, 3241 cm-1 is assigned to the N-H, 1674 cm-1 is assigned to the C=O, 1274 cm-1 is assigned to the C=N, 590 cm-1 is assigned to the C- H in the complex. The electronic spectra of the synthesized compound, has been recorded by using the spectrometer in the range of 200-600 nm. The 1H-NMR spectra of NABA Ligand displays a signal at 7.4 ppm which is assigned to protons of benzen’s beta, one signal at 8.8 ppm which is assigned to protons of a-pyridil ring and a signal at 7.6ppm which is assigned to protons of C=O.
References
- Jeeworth T., Wah H. L. K., Bhowon M. G., Ghoorhoo D., Babooram K. Synt. React. Inorg. Met-Org. Chem., 30: 1023 (2000).
- Dharmaraj N., Viswanalhamurthi P., Natarajan K. Trans. Met. Chem., 26: 105 (2001).
CrossRef
- Yildiz M., Dulger B., Dulger S.-Y., Koyuncu B.-M. J. Indian Chem. Soc., 81: 7 (2004).
- Lipscomb W.-N., Strater N. Chem. Rev. 96: 2375(1996).
CrossRef
- Cozzi P. G. Chem. Soc. Rev., 33: 410 (2004).
CrossRef
- Vallee B.-L., Auld D.-S., Acc. Chem. Res. 26: 543 (1993).
CrossRef
- Jayabalakrishnan C., Natarajan K. Synt. React. Inorg. Met-Org. Chem., 31: 983 (2001).
- Chandra S., Sangeetika J. Indian Chem., Soc. 81: 203 (2004).
- Maiti A., Ghosh S. Indian J. Chem., 28: 980 (1989).
- Aggarwal R. C., Singh N. K., Singh R. P. Inorg. Chim. Acta., 29: 2794 (1981).
CrossRef
- Sun X.-X., Qi C.-M., Ma S.-L., Huang H.-B., Zhu W.-X. Liu Y.-C. Inorg.Chem.Commu. 9: 911(2006).
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal