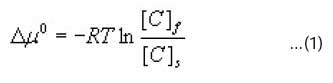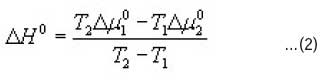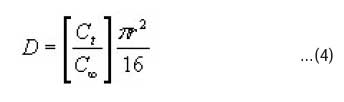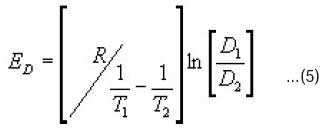Thermodynamic Absorption Parameters of Disazo Dyes derived from P-aminophenol and 4-Aminobenzoic Acid on Polyester Fibre and Nylon 6 Fibre
J. O. Otutu
Department of Chemistry, Faculty of Science, Delta State University, PMB 1, Abraka, Delta State, (Nigeria).
DOI : http://dx.doi.org/10.13005/msri/090107
Article Publishing History
Article Received on : 25 Apr 2012
Article Accepted on : 28 May 2012
Article Published :
Plagiarism Check: Yes
Article Metrics
ABSTRACT:
The thermodynamic absorption parameters of a series of disazo dyes derived from paminophenol and 4-aminobenzoic acid on polyester and nylon 6 substrates are described. It was found that the dyes generally have higher affinities for the polyester fibre than for the nylon 6 fibre. The values of Δm0, ΔH0, and ΔS0 in the two dye-fibre systems suggest that the hydrogen bonding mechanism is operative in the dye-fibre binding forces. The very high values of correlation coefficients showed that the rate curves fit the experimental data perfectly. The results of the study also showed that certain intrinsic properties such as planarity and molecular volume could be more useful in interpreting activation energy of diffusion data.
KEYWORDS:
Disazo dyes; diffusion coefficient; Activation energy; polyester and nylon 6 fibres
Copy the following to cite this article:
Otutu J. O. Thermodynamic Absorption Parameters of Disazo Dyes derived from P-aminophenol and 4-Aminobenzoic Acid on Polyester Fibre and Nylon 6 Fibre. Mat.Sci.Res.India;9(1)
|
Copy the following to cite this URL:
Otutu J. O. Thermodynamic Absorption Parameters of Disazo Dyes derived from P-aminophenol and 4-Aminobenzoic Acid on Polyester Fibre and Nylon 6 Fibre. Mat.Sci.Res.India;9(1). Available from: http://www.materialsciencejournal.org/?p=1174
|
Introduction
Dyeing is a process not only of mass transfer but also one in which interaction in different degree between dye and fibre take place. Thus, the inclusion of thermodynamic sorption data is essential1,2. The application of physical chemistry to dyeing process led to the concept of “dyeing affinity”. That is the free-energy change accompanying dye adsorption process, as a measure of the strength of dye-fibre bonding3,4. The standard heat of dyeing, ΔH0 represents the total change in enthalpy of the system during dyeing as a result of molecular interactions at any point within the system. Both the solution phase and the fibre phase contribute to DH0. And from the values of ΔH0, one hopes to learn something of the values of the forces of attraction between dyes and fibres and the manner in which these forces of attraction between dyes and fibres are influenced by the chemical structure of the dyes and the fibre; since ΔH0 is influenced by every single molecular interaction both in the solution and in the fibre phases5,6,7. Similarly, the standard entropy of dyeing ΔS0, is a much more subtle aspect of the dyeing process that has to do with the ordering or the dispersal of molecules within the system.
In a study involving a fabric blend, the kinetics of dyeing process was reported of polypropylene polyster fibre blends using disperse dye C. I. Disperse Blue 568. Wherein the dye uptake, dyeing rate constants, diffusion coefficients and activation energy of PP/PET blend were investigated.
In another study, the thermodynamic sorption parameters of 1,4-diaminoanthraquinone on polyethylene terephthalate using several alkane media ranging from pentane to decane was reported9. They found that in the range from pentane to decane, as the number of carbon atoms increases, the standard affinity (-Δm0) decreased. Also the diffusion coefficient (D) of the dye decreased and the activation energy (ED) increased with increasing number of carbon atoms in the alkane.
Although, many papers described the thermodynamic parameters of monoazo dyes and other dye types on cellulose, proteins and polyamide fibres and to some extent on polyester fibres, very few comparable investigations have been made with disazo disperse dyes on synthetic polymer – fibres. Hence, in this present study, the thermodynamic absorption parameters of disazo disperse dyes prepared in [10] are described. We also examined the kinetics of the dyes on nylon 6 substrate.
Preparation of dye dispersion
The previously prepared dyes as described in the above sub-section were formulated to be applied to PET and nylon 6 fabrics. The composition of the dye dispersions comprised dispersing agent. Diwatex 40P (moderately sulphonated Kraft lignin, 20% on the weight of the dye) and N, N-dimethylformamide (DMF). The dispersions were prepared by dissolving each dye (0.40 g) in 8 mL of DMF and adding dispersing agent to the mark (100 mL).
Determination of Standard Affinity, Heat of Dyeing and Entropy of Dyeing.
Nine tightly closed test-tubes were placed in a thermostated dyebath-set at 363 K and 373 K respectively. Ten milliliters of dye liquor was placed in each test-tube to give 10 percent shade with a liquor ratio 80:1. Absorbance readings were taken on a camspsec spectrophotometer. 0.04 g of polyester (terelyene) fabric (100%) sample obtained from Multichem (Nigeria) which was not pretreated, was placed in each test-tube and covered. Timing started when the temperature of the dye liquor equilibrated with the dyebath and the bath was left to stand for six hours. After, the concentration of dye, [C]s, remaining in the dyebaths was measured in a 10 mm cell by the spectrophotometric method. The dyed samples were rinsed thoroughly with deionised water. The above procedure was repeated for the dyeing of nylon 6 fabrics at 353 K, and 363 K respectively at pH 4, adjusted with 2% acetic acid.
The standard affinity Δμo, (J/mol) measured at 363 k and 373 k was calculated using Eqn 111.
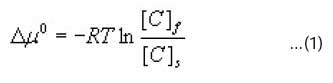
Where R is the gas constant (8.317 J/mol K), T the absolute temperature (K), [C]f the concentration of the dye on the fibre at equilibrium (g/kg), [C]s, the concentration of dye in the dyebath at equilibrium (g/L).
The heat of dyeing ΔHo (J/mol) was calculated in accordance with Eqn 212, 13.
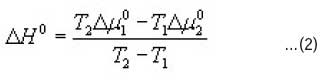
Were T1 and T2 are the absolute temperatures 1 and 2 and Δμo 1 and Δμo 2 (J/mol) are the standard affinities at T1 and T2 respectively.
Finally, Eqn 3 was used to find the entropy of dyeing, ΔSo (J/mol)14.
Δμo
Δμ0= ΔHo – TΔSo …(3)
Determination of diffusion (Sorption) coefficient and Activation energy of diffusion
Nine identical dyebaths (round bottomed flasks) were prepared and eleven 0.05g nylon 6 fabric samples were used. Dye dispersion was placed in each flask to give 20 percent shade at a liquor ratio 20:1. The dyeing system was maintained at pH 4.0 using 2% acetic acid.
The nylon 6 fabrics were entered into each flask. The flasks were placed in a thermostated dyebath set at 343 K and 353 K respectively. Timing started when the temperature of the dye liquor equilibrated with that of the dyebath, and a fabric sample was removed from the dyebath at intervals of 10min and the last sample was removed after six hours of dyeing. Each dyed sample was washed with warm water and allowed to dry at room temperature. The absorbances of the dyes were
measured after striping the dyed fabric samples with formic acid at λmax (400 nm) in a 10 mm quartz absorption cell campsec uv/vis spectrophotometer. The absorbance at 10min intervals were [C]t, and the absorbance after six hours of dyeing were [C]¥. All measurements of dye solution were conducted at room temperature and graphs of Ct/C¥ against t1/2 were plotted from where the slopes were obtained. The diffusion coefficients (D) were calculated using Eqn 415.
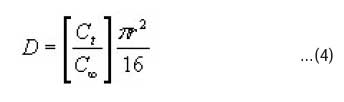
The activation energy of diffusion ED was calculated using Eqn 516.
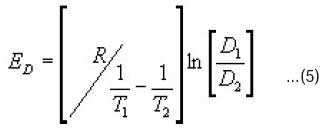
Note that the difference in the temperatures of study for polyester and nylon is due to the type of fibre. For example, polyester is more crystalline in structure than nylon hence it requires higher temperatures for dyeing to take place.
Results and Discussion
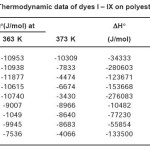
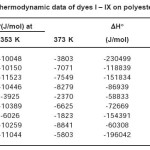
Tables 1 and 2 show the results of thermodynamic studies including standard affinity, heat of dyeing and entropy of dyeing while figures 2 to 10 gives the rate curves of Dye I to IX correspondingly. The standard affinity of the dye samples decreased in magnitude with temperature, indicating that the migration of dye from the dyebath to the fibre was not enhanced. This is so because in most dye-fibre systems, the enthalpy of dyeing is negative. As a result, the magnitude of the standard affinity at 373 K was less than that at 363 K for the polyester dyeing system and also the magnitude of the standard affinity at 363 K was less than that at 353 K for the nylon 6 dyeing system. The standard heat of dyeing, ΔHo, is of practical significance because it provides a quantitative description of the influence of temperature on dyeing equilibrium. The heat of dyeing is regarded as the sum of heats of formation of the various bonds, such as hydrogen bonds and ionic bonds, holding dye and fibre
together. A decrease in the value of ΔHo means that more dye can be retained in the fibre, that is the interaction between dye and fibre is increased.
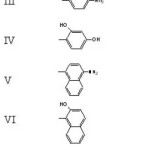
Figure 1: Structure of Dyes
Table 1: Thermodynamic data of dyes I – IX on polyester fabrics
Table 2: Thermodynamic data of dyes I – IX on polyester fabrics
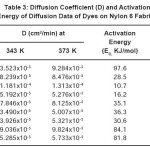
Table 3: Diffusion Coefficient (D) and Activation Energy of Diffusion Data of Dyes on Nylon 6 Fabrics
When dye molecules migrate from the solution (or dispersion) to the fibre in a dyeing system, they become more ordered due to the confinement in the solid fibre phase. The results in Table 1 also show that the heat evolved on dyeing is quite high for both substrates and the dyes which gave decreased enthalpy of dyeing were more than compensated for by the corresponding increase in entropy. For instance, dye (II) in the polyester dyefibre system gave a decreased enthalpy of -9007 J/ mol but its entropy increased to -4J/mol.
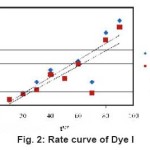
Figure 2: Rate curve of Dye I
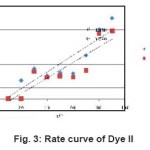
Figure 3: Rate curve of Dye II
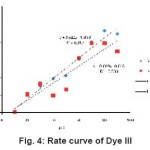
Figure 4: Rate curve of Dye III
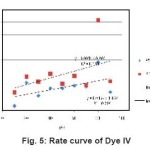
Figure 5: Rate curve of Dye IV
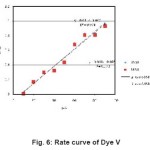
Figure 6: Rate curve of Dye V
Figure 7: Rate curve of Dye VI
Similarly, in dye (V), the nylon 6 dye-fibre system gave a decreased heat of dyeing of -58833 J/mol and a corresponding increase in entropy of – 136 J/mol. This tends to corroborate the recent work done on the physico-chemical properties of monoazo dyes on synthetic-polymer-fibres10. However, the values of Δμo for the PET dye-fibre system are higher than those of the nylon 6 dyefibre system except dyes (VI), (VII) and (IX). This suggests that the dyes generally have higher affinities for the polyester fibre than for the nylon 6 fibre.
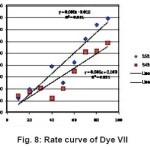
Figure 8: Rate curve of Dye VII
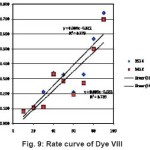
Figure 9: Rate curve of Dye VIII
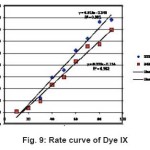
Figure 9: Rate curve of Dye IX
The values found for the diffusion coefficient (Table 3) on nylon 6 fibre show that the higher the temperature, the higher the coefficient of diffusion. This indicates that at high temperatures, there is greater loosening up of the fibre structure, thus creating more spaces for easier diffusion of dye molecules.
Activation Energy of Diffusion (ED)
The values found for the activation energy of diffusion of each dye sample on nylon 6 fibre are given in Table 3. Here the values of ED reflect the amount of energy needed to assist the dye molecule in interring the fibre so as to overcome the surface barrier of the fibre. A small value means that the dye requires relatively little energy to overcome the surface barrier before entering the fibre while a larger value is the reverse7. It was also found that dye (I) has the highest value of ED followed by dye (VI) and (VII) respectively. These dye molecules must have entered the nylon 6 fibre with more difficulty than the others. This suggests that certain factors such as molecular weight can influence or affect dye diffusion and hence activation energy of diffusion. Comparing dyes (VI) and (VII) on molecular weight factor, it was observed that the latter has smaller value of activation energy of diffusion than the former as the molecular weight is lower. Similarly, dye (I) has higher activation
energy of diffusion than dye (II) yet its molecular weight is lower. But this is not so when dyes (I) and (VI) are compared. For example, dye (I) has the highest activation energy of diffusion value of 97559 J/mol-1 and molecular weight of 351.8 while dye (VI) has a lower ED and yet its molecular weight is higher. This suggests that other intrinsic properties of the dye molecules such as planarity and molecular volume apart from molecular weight could be very important in analyzing activation energy of diffusion data. These agreed with that reported in the literatures14,16.
Conclusion
The dyeing properties of a series of disazo dyes were studied thermodynamically and kinetically on PET and nylon 6 substrates. In the thermodynamic study, it was found that the dyes generally have higher affinity for the polyester fibre than for the nylon 6 fibre. On the other hand, there were changes in the ΔHo and ΔSo in the two dyefibre systems. The values of the ΔSo were negative which do not favour dyeing. However, the ΔHo values were higher which suggests that it is the only driving force for dyeing to occur. The values of the Δμo, ΔHo, and ΔSo and also the presence of the –OH and –NH2 in the dye structures indicate that the hydrogen bonding mechanism is involved in the dye-fibre binding forces. The values of activation energy of diffusion did not show any general trend, however, certain intrinsic properties of the dye molecules such as planarity and molecular volume could be more useful in interpreting activation energy of diffusion data for nylon 6 substrate. Further work in this area and that of the kinetics of PET fibre could prove to yield beneficial results.
References
- Venkataraman, K., The Chemistry of Synthetic Dyes. New York, Academic Press, (1974).
- Otutu, J. O., Ossai, E. K., Jatto, E. O., Synthesis and Physicochemical Properties of monoazo disperse dyes from 3-aminophenol. J. Chem. Soc. Nig. 32(2): 81-99 (2007).
- Bird, C. L. & Boston, W. S., The Theory of Coloration of Textiles. Society of Dyers and Colourists, Bradford, (1975).
- Abrahavt E. V., Dyes and Their Intermediates. London. Edward Arnold Pub. Ltd, (1977).
- Mcgregor, R., Theory of Dyeing. Journal of Society of Dyers and Colourists, 83(1): 57- 58 (1967).
- Daruwalla, E. H. in The Chemistry of Synthetic Dyes Eds. Venkatavaman, New York, Academic Press, (1974).
- Kan, C. W., Chan, K., Yuen, C. W. M. and Miao, M. H., Plasma modification in Wool fibre Effect on the dyeing properties. Journal of Society of Dyers and Colourists, 114(5): 61- 65 (1998).
CrossRef
- Anna, U., Eva, B., Janka, O., Randovan, T. and Anton M., Kinetics of dyeing process of blend polypropylene/polyester fibres. Dyes and pigments, 72(2): 212-216, (2007).
CrossRef
- Kim, T., Son, Y., Lim, Y., Thermodynamics analysis of 1, 4-diaminoanthraquinone adsorption on polyethylene terephthalate in alkane media; Dyes and pigments, 72(2): 246 – 250, (2007).
CrossRef
- Otutu, J. O., Okoro, D. and Ossai, E. K., Preparation of Dis-azo dyes derived from paminophenol and their fastness properties for synthetic polymer-fibres. J. Appl. Sci. 8(2): 334-339 (2008).
CrossRef
- Giles, C. H., Laboratory Course in Dyeing, Pp. 134, (1974).
- Peters, L., In The Theory of Coloration of Textiles; Eds. Bird C. L. and Boston W. S. Society of Dyers and Colourists, Bradford, (1975).
- Giles, C. H., Laboratory Course in Dyeing, Bradford, Soc. Dyers and Colourists (SDC) Pp. 138, (1974).
- Yakubu, M. K., Ph.D Thesis, Ahmadu Bello University, Zaria, Nigeria, (2000).
- Venkataraman, K., The Chemistry of Synthetic Dyes: New York Academic Press, Pp. 131-132, (1974).
- Otutu, J. O., PhD Thesis, University of Benin, Benin City, Nigeria, (2005).

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal