X-ray diffraction Studies of Cu(II), Zn (II), Mo(II), Fe(II)complexes with Glibenclamide (5-chloro-N-(4-[N-(Cyclohexyl Carbnonyl) sulfamonyl]phenethyl)-2-methoxylbenzamide
Mohammad Tawkir1*, Balkrishan1, S. A. Iqbal2 and Ishaq Zaafarany
¹Deparment of Chemistry, Saifia College of Science, Bhopal - 462 001 (India).
²Deparment of Chemistry, Crescent College of Technology, Bhopal - 462 038 (India).
³Deparment of Chemistry, Material Science Umm- Al-Qura University, Makkah-Al-Mukaramah (Saudi Arabia).
DOI : http://dx.doi.org/10.13005/msri/090112
Article Publishing History
Article Received on : 20 Mar 2011
Article Accepted on : 07 May 2011
Article Published :
Plagiarism Check: No
Article Metrics
ABSTRACT:
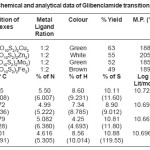
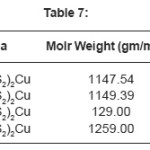
Glibenclamide (5-chloro-N-(4-[N-(cyclohexyle carbnonyl) sulfamonyl]phenethyl)-2- matyhoxylbenzamide was used to synthesize Cu(II), Zn (II), Mo(II), Fe(II)complexes. Metal Complex were characterized by elemental analysis. IR, TGA, The Crystal structure of complexes were further determined by X-ray diffraction method. The XRD data was used to index the compound for tetragonal and octahedral system.
KEYWORDS:
Glibenclamide; Crystal structure; Cu(II); Zn (II); Mo(II); Fe(II) complexes
Copy the following to cite this article:
Tawkir M, Balkrishan, Iqbal S. A, Zaafarany I. X-ray diffraction Studies of Cu(II), Zn (II), Mo(II), Fe(II)complexes with Glibenclamide (5-chloro-N-(4-[N-(Cyclohexyl Carbnonyl) sulfamonyl]phenethyl)-2-methoxylbenzamide. Mat.Sci.Res.India;9(1)
|
Copy the following to cite this URL:
Tawkir M, Balkrishan, Iqbal S. A, Zaafarany I. X-ray diffraction Studies of Cu(II), Zn (II), Mo(II), Fe(II)complexes with Glibenclamide (5-chloro-N-(4-[N-(Cyclohexyl Carbnonyl) sulfamonyl]phenethyl)-2-methoxylbenzamide. Mat.Sci.Res.India;9(1). Available from: http://www.materialsciencejournal.org/?p=1188
|
Introduction
Polyfunctionally rings compounds and synthesis of their metal complexes which have various biological activities and include hetero atom, have been formed in organic synthesis and coordination chemistry.1-3 Many trasition and inner trasition metal complexes have been synthesized for analytical and commercial applicatisis many of medicinal use.4-5 Literature survey reveals that the transition metal complexes generally crystallized with tetrahedral, octahedral geometry.6-3
Table 1: Physico-Chemical and analytical data of Glibenclamide transition metals complexes
Experimental
All the chemicals used for the preparation of complexes are of Hi media AR grade E-merk. Metal Complexes are synthesized by adding metal salt solution in appropriate solvent to the solution of the ligand. The mixture was refluxed for 3-4 hours. Then the precipitate of metal complexes was obtained. It is filtered, washed and dried in vaccum desiccators.
All metals forms 1:2 complexes with Glibencalmide were confirmed by to methods as modified and Anderson.9-11
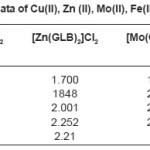
Table 2: ESR data of Cu(II), Zn (II), Mo(II), Fe(II) complexes
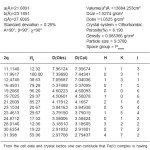
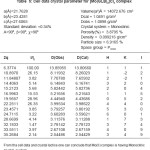
Table 3: Cell data and crystal parameters for [Cu(GLB)2]CI2 complex
Results And Discussion
The Results of ESR spectra and X-ray diffraction of Cu(II), Zn (II), Mo(II), Fe(II) complexes with Glibencalmide were obtained and summarized in following Tables. All reflections has been indexed for h,k, 1 values using reported literature12-16 and full proof suit XRD software v.2.0 by using fool proof suite XRD software the d-values of metal complexes were obtained. From ESR spectra of complexes the value of g1,g2,g3 and gav can be determined. These values are tabulated in Table 2.
In case of CU(II) complexes gav value is 2.09 which is less than 2.271, the values of indicates the presence of sufficient covalence between the metal ion and donar atom.17 In case of Zn(II) complex gav value is found to be 2.21 this value is less than 2.25, it is assignable to the presence of covalent character in metal ion donar atom. In case of Mo(II) complex and Fe(II) complex gav values found to be 2.30 and 2.09 respectively. This values less than 2.247 indicates presence of covalent characters in coordinate bond.
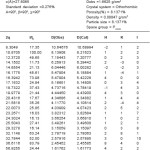
Table 4: Cell data crystal parameter for [Zn(GLB)2]CI2 complex
Table 5: Cell data crystal parameter for [Mo(GLB)2]CI2 complex
Table 6: Cell data crystal parameter for [Fe(GLB)2]CI2 complex
X-Ray Diffraction Of Cu(II) Compex
The X-ray diifraction pattern of Cu(II), Zn(II), Mo(II) and Fe(II) complexes has been determined 20 range from 5.0084 to 79.97884 and data has been summarized in the following table.
Conlusion
On the basis of magnetic moment, crystal lactice parameters and spectral data complexes of Cu(II), Zn(II) exhibit tetragonal structure were as Mo(II) and Fe(II) complexes exhibit octahedral structure.
The proposed structure of Cu (II), Zn (II), Mo (II) and Fe (II) complexes as shown in the following figure.
Acknowledgements
The author is thankful to the principal of Saifia Science College, Bhopal and Principal of Cresent Engineering College, Bhopal for providing all necessary facilities and Punjab University for providing XRD spectra.
References
- S.N Pandeya, D. Sriram, G. Nath and E.De. Clereq, Arzneim-forsch/Drug Res. 50-55 (2000).
- B.S. Hollo, K.A. Poojary and B. Kollurya, II Farmaco, 51: 793 (1996).
- M. Kidwai, P. Sapra, P. Mishra, R.K. Saxena and M. Singh. Bioorg. Med. Chem. 9: 217 (2001).
CrossRef
- B.H. Mehta and S.A. Dugaonkar, Proceeding of 13th, Australia Symposium on analytical chemistry, Darwin Northern, Australia (1995).
- S.A. Dagaonkar, Ph. D. Thesis. University of Mumbai, India (1995).
- S. Prakash, Y. Dutt and R.P. Singh, Indian J. Chem. 7: 512 (1969).
- R.P. Bhargava and M. Tyagi, Indian 25A: 193 (1986).
- N.S. Bhave, R. B. Kharat. J. Chem. Soc. 56: 244 (1979).
- Mamta Bhattacharya, S.A. Iqbal and Suman Malik, Orient J. Chem. 20(4): 643-646 (2004).
- Asmi Denavi and Iqbal S.A., Orient J. Chem.2(2): 156-159 (1986).
- N.F.M. Henry, H.Lipson and W.A. Wooster Interpretation of X-ray diffraction photograph 81 (1851).
- Chohan, Zahid H. Praveen M. and Ghaffer A., Synth. React. Inorg. Met. Org. Chem. 28(1): 1973 (1998).
- Juan Rodriguez-Carvajal and Institute Lue Langevin Diffraction group 6, rue, Jules Horowitz B.P. 156-3804.
- Altermatt. D. and Brown, I.D. Acta. Cyst. B41:244-247 (1985).
- Brown, I.D. The Chemical bond in inorganic chemistry, The bond balance model IUCr monographs on crystallography, 12, Oxford University Press, (2002). www.ccp14.ac.uk/ ccp/web-mirros/idbrown.
- R.L. Dutta and A. synmal, Elements of mangetochemistry, Affiliated East-West Press. Pvt. Ltd. Edn.: 2 (1993).

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal