Preparation and Characterization of the Schiff Base Ligand (E)- N-(3-nitrobenzylidene) isonicotinohydrazide and its Silver (I) Complex [Ag(NNBIH)2]+
Shahriar Ghammamy1* and Sajjad Sedaghat
Department of Chemistry, Faculty of Science, Islamic Azad University, Malard Branch, Malard, Iran
DOI : http://dx.doi.org/10.13005/msri/090203
Article Publishing History
Article Received on : 20 Feb 2012
Article Accepted on : 25 Mar 2012
Article Published :
Plagiarism Check: No
Article Metrics
ABSTRACT:
We describe the synthesis and characterization of a new silver (I) complex of the Schiff base ligand (E) - N-(3-nitrobenzylidene) is onicotinohydrazide that abbreviated as NNBIH was synthesized and characterized. Ag (I) Metal complex of this Schiff base ligand prepared by reaction of nitrate salt of Ag (I) with NNBIH. Characterization of the ligand was made by microanalyses, FT-IR, UV–Visible spectroscopy and its complex was made by microanalyses, FT-IR and UV–Visible spectroscopy. TG- DTA and other analytical methods have been applied to the investigation of the thermal behavior and Structure of the compound [Ag(NNBIH)2]+. Thermal decomposition of these compounds is multistage processes.
KEYWORDS:
(E)-N-(3-nitrobenzylidene) isonicotinohydrazide; Ag(I) complex; Synthesis; Thermal analysis
Copy the following to cite this article:
Ghammamy S, Sedaghat S. Preparation and Characterization of the Schiff Base Ligand (E)- N-(3-nitrobenzylidene) isonicotinohydrazide and its Silver (I) Complex [Ag(NNBIH)2]+. Mat.Sci.Res.India;9(2)
|
Copy the following to cite this URL:
Ghammamy S, Sedaghat S. Preparation and Characterization of the Schiff Base Ligand (E)- N-(3-nitrobenzylidene) isonicotinohydrazide and its Silver (I) Complex [Ag(NNBIH)2]+. Mat.Sci.Res.India;9(2). Available from: http://www.materialsciencejournal.org/?p=1101
|
Introduction
Schiff bases have often been used as chelating ligands in the field of coordination chemistry and their metal complexes are of great interest for many years. It is well known that N and S atoms play a key role in the coordination of metals at the active sites of numerous metallobio molecules.1 Schiff base metal complexes have been widely studied because they have industrial, antifungal, antibacterial, anticancer and herbicidal applications.2-3 They serve as models for biologically important species and find applications in biomimetic catalytic reactions. Chelating ligands containing N, S and O donor atoms show broad biological activity and are of special interest because of the variety of ways in which they are bonded to metal ions. It is known that the existence of metal ions bonded to biologically active compounds may enhance their activities.4-6 The variety of possible Schiff base metal complexes with wide choice of ligands, and coordination environments, has prompted us to undertake research in this area.7 Zinc can function as active site of hydrolytic enzymes, where it is ligated by hard donors (N or O). It has long been recognized as an important co-factor in biological molecules, either as a structural template in protein folding or as a Lewis acid catalyst that can readily adopt the coordination numbers 4, 5, or 6.8-10 The catalytic role of Zn comprises Lewis acid activation of substrate, generation of a reactive nucleophile (Zn- OH) and stabilization of the leaving group. As a part of our continuing work on dissymmetric tetradentate Schiff base complexes containing N, S and O donor atoms11 and in light of the importance of Cd and Zn ion metals, recently, there has been a considerable interest in the chemistry of hydrazide compounds because of their potential pharmacological applications. Several reports on synthesis of metal complexes of hydrazide by the use of organic solvents under reflux and their biological properties have already been published. More so, these methods suffer several drawbacks such as long reaction time, an excess of organic solvent, lower products yield and harsh refluxing conditions. We now report the synthesis and characterization of Ag (I) complex of the Schiff base ligand (E)-N-(3-nitrobenzylidene) isonicotinohydrazide.
Material and Methods
All reagents were supplied by Merck and were used without further purification. Melting point was determined in an Electrothermal 9200. The FTIR spectra were recorded in the range 400–4000 cm-1 byKBr disk using a Bruker Tensor 27 M 420 FT-IR spectrophotometer. The UV–Vis spectra in CH3CN were recorded with a WPA bio Wave S2 100 spectrophotometer.Elemental CHN analyses were performed using a Heraeus CHN-O-RAPID elemental analyzer. Thermogravimetric analyses were done on a Perkin Elmer TGA/DTA lab system l (Technology by SII) in nitrogen atmosphere with a heating rate of 20°C/min from 35-700°C.
Synthesis of the NMBIH Ligand
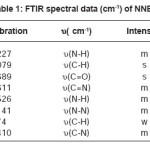
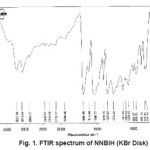
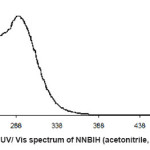
For synthesis of the NNBIH ligand, to a magnetically stirred of 4-Pyridinecarboxylic acid hydrazide (1.371 g, 1mmol) in hot methanol (20 ml) was added 3-nitrobenzaldehyde(0.151g, 1mmol) via a syringe and heated for 4 h. After cooling to room temperature, the resulting white precipitate was filtered, washed with n-hexane (20 ml) and dried.Mp215-217º CYield: 79%. Anal. Calc. for C14H11N3O3: C, 62.45; H, 4.08; N, 15.61%. Found: C, 62.58; H, 4.20; N, 15.77%. IR (KBr Disk, cm-1): 3227 (m, N-H), 3079(s, C-H); 16771689 (m, C=O); 1611 (m, C=N); 674 (w,CH3). UV–Vis, λ max (ε) = 271 (1872), 292(2282) (Fig. 1, 2, 3, Table 1).
Preparation of [Ag(NNBIH)2]+
NNBIH(0.269g, 1mmol) and AgNO3 (0.0845g, 0.5 mmol) were dissolved at room temperature. The mixture was stirred and heated for 4 h to give a clear solution. After cooling to room temperature, the resulting yellow precipitate was filtered, washed with n-hexane (20 ml) and dried. Mp220-2220C Yield: 82%. Anal. Calc. for C26H18N8O6Ag: C, 48.30; H, 2.78; N, 17.34%. Found: C, 48.58; H, 2.95; N,17.55%. FTIR (KBr pellet, cm-1): 3441(m, CH), 424(w,Ag-N); 1680 (s, C=N), 1615 (s, C=C), 1282 (m, C-O), 1070 (s, C-H), 1615(s, C=C). UV–Vis, λ max (nm)/ε (M-1cm-1); 294 (2968). (Fig. 4, Table 2).
Results and Discussion
In this study we have reported the synthesis of a new hydrazide derivative and it, s Ag (I) complex. The structural characterizations of synthesized compounds were made by using the elemental analysis, IR and UV spectral techniques. From the spectroscopic characterization, it is concluded that ligands act as a neutral bidentate through the azomethine nitrogen atom and carbonyl groups.
Synthesis and Stability
Ag (I) salt react with Schiff base ligand in 2:1(L/M) molar ratio in solvent to afford complex. The ligand and its complex are stable at room temperature. These NNBIH ligand and (E) – N-(3- nitrobenzylidene) isonicotinohydrazideSilver (I) compound were obtained in relatively high yield, 79 and 82% respectively.These title compounds are soluble insolventssuch as methanol, ethanol, acetonitrile, DMSO, acetoneandchloroform, less soluble in intolueneandpetroleumether and in
soluble in water, etherandhexane too. The strong bands at 3079 and 1689 cm-1 are assigned to the C-H and -C=O- stretching vibrations compound in NNBIH ligand, 1680 cm-1 is assigned to the C-N, 1615 cm-1 is assigned to the C=C and 1070 cm-1 is assigned to the C-H in the complex. The electronic spectra of the synthesized compound, has been recorded by using the spectrometer in the range of 200-600 nm.
Table 1: FTIR Spectral Data (cm-1) of NNBIH
Table 2: FTIR Spectral Data (cm-1) of [Ag(NNBIH)2]+
Figure: 1 FTIR spectrum of NNBIH (KBr Disk)
Figure 2: UV/ Vis spectrum of NNBIH (acetonitrile, 5×10-4 M)
Figure 3: 1H-NMRspectrum of [Ag(NNBIH)2]+ (in CDCl3)
Figure 4: UV/ Vis spectrum of [Ag(NNBIH)2]+ (acetonitrile, 5×10-4 M)
Figure 5: Thermal analysis data of [Ag(NNBIH)2]+
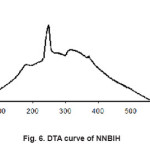
Figure 6: DTA curve of NNBIH
Thermogravimetric Analyses
The thermal properties of these compounds were investigated by thermograms (TG, DTG and DTA). Fig. 5 shows TGA and DTA curves for[Ag(NNBIH)2]+. In the temperature range 40-165 °C, 3.6% weight losing was observed which was related to the loss ofmost parts of compound. In the temperature range from 165-230°C, 10.3% weight reduction was found, which was related to the loss of another part of compound. In the temperature range from 230-310°C, 74.4% weight reduction was found, which was related to the loss of another part of compound and the latest weight losing found in the 310-600°C temperature range in that 7.7% of the sample weight decreased that is related to lose of other part of the compound. Fig. 6 curves of differential thermal analysis (DTA) shows. In this method, phase changes or chemical reaction scan be tailored to the study of heat absorbed or released. This method for assessing changes in solids at high temperatures is suitable. Since the normal to the sample is heated, Endo thermic and exothermic processes occur during the heating of the sample. Exothermic processes are observed when by secondary reactions redecoration take place. NNBIH in the DTA curve Endothermic and exothermic process is observed. The endothermic process occurs at 180, 250 and 315C°. Heating processes are related to the formation of new bonds namely after passing through the broken bond sand endothermic process, due to redecoration and new bonds formed, heat is released in the environment. (The bond is associated with energy release). (Fig. 5, 6).
Acknowledgment
We gratefully acknowledge the financial support from the Research Council of malard Islamic Azad University and many technical supports that provided by TarbiatModarres University.
References
- Singh K.and Barwa, M.S., Eur. J. Med. Chem., 42: 394 (2007).
CrossRef
- Cozzi P.G.,Chem. Soc. Rev., 33: 410 (2004).
CrossRef
- Chandra S.,J. Indian Chem. Soc., 81: 203 (2004).
- Ferrari, M.B.,Capacchi, S., Pelosi, G.,Reffo, G.,Tarasconi, P.,Albertini, R.,Pinelli,
CrossRef
- S.,Lunghi, P. Inorg. Chim. Acta., 286: 134 (1999).
- Canpolat E., Kaya M., Chem. J. Coord., 57: 1217 (2004).
CrossRef
- Yildiz M., Dulger B., Dulger S.Y.,Koyuncu, B.M., J. Indian Chem. Soc., 81: 7 (2004). Majumder A., Rosair G.M., Mallick A.,Chattopadhyay N., Mitra S., Polyhedron., 2006; 25: 1753 (2006).
- Lipscomb W.N., Strater N.,Chem. Rev., 96: 2375 (1996).
CrossRef
- Vallee B.L., Auld D. S., Acc. Chem. Res., 26: 543 (1993).
CrossRef
- Sun X.X., Qi C.M., Ma, S.L., Huang H.B., Zhu W.X., Liu Y.C., Inorg. Chem. Commu., 9: 911 (2006).
CrossRef
- Saghatforoush, L. A., Hossaini Sadr M., Lewis W.,Wikaira J., Robinson, W.T., and Ng, S.W., Structure Reports Online., 60: 1259 (2004).
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal

![Table 2: FTIR spectral data (cm-1) of [Ag(NNBIH)2]+](http://www.materialsciencejournal.org/wp-content/uploads/2012/12/Vol.09_No2_Pre_SHA_T2-150x150.jpg)
![Fig. 3.1H-NMRspectrum of [Ag(NNBIH)2]+ (in CDCl3)](http://www.materialsciencejournal.org/wp-content/uploads/2012/12/Vol.09_No2_Pre_SHA_fig3-150x150.jpg)
![Fig. 4. UV/ Vis spectrum of [Ag(NNBIH)2]+ (acetonitrile, 5×10-4 M)](http://www.materialsciencejournal.org/wp-content/uploads/2012/12/Vol.09_No2_Pre_SHA_fig4-150x150.jpg)
![Fig. 5. Thermal analysis data of [Ag(NNBIH)2]+](http://www.materialsciencejournal.org/wp-content/uploads/2012/12/Vol.09_No2_Pre_SHA_fig5-150x150.jpg)