Flame Retardant Polyurethanes and their Applications for the Improvement in Properties of Conventional Castor Oil Based Polyurethane
Rasmika H. Patel1*, Amin V. Hirani1, Hitesh B. Patel2
1Department of Materials Science, Sardar Patel University, Vallabh Vidyanagar - 388 120. Gujarat, India.
2Gujarat Narmada Valley Fertilizers Company Limited, Narmada nagar -392 015. Bharuch, Gujarat, India.
DOI : http://dx.doi.org/10.13005/msri/110209
Article Publishing History
Article Received on : 28 Nov 2014
Article Accepted on : 06 Dec 2014
Article Published : 10 Dec 2014
Plagiarism Check: Yes
Article Metrics
ABSTRACT:
The urethane polymers of castor oil (COPU) are used in many commercial applications but one of the major limitations of these polymers is their inherent flammability and poor mechanical properties. Polymers containing phosphorous groups belong to the fire retardants, and when blended with castor oil based polyurethane improves the latter’s fire retardant properties. Present work, deals with the synthesis of a monomer bis (m-hydroxy phenyl) phenyl phosphate (BHPPP) and its related polyurethanes. Characterization of the monomer and polyurethanes are discussed. Blending of the polyurethanes with COPU in different proportions improves both the flame retardant and mechanical properties of the COPU.
KEYWORDS:
polyurethanes; flame Retardant; film; coating; properties
Copy the following to cite this article:
Patel R. H, Hirani A. V, Patel H. B. Flame Retardant Polyurethanes and their Applications for the Improvement in Properties of Conventional Castor Oil Based Polyurethane. Mat.Sci.Res.India;11(2)
|
Copy the following to cite this URL:
Patel R. H, Hirani A. V, Patel H. B. Flame Retardant Polyurethanes and Their Applications for the Improvement in Properties of Conventional Castor Oil Based Polyurethane. Mat.Sci.Res.India;11(2). Available from: http://www.materialsciencejournal.org/?p=921
|
Introduction
Polyurethanes are used in versatile applications due to their properties like toughness, flexibility, physical, mechanical and chemical resistancy1,2. Synthesis of phosphorus based flame retardant polyurethanes opens another area of their use specifically in the surface coating application where flame retardancy is required3-5. Present work deals with the synthesis of intrinsic type of flame retardant polyurethanes and their characterization using various chemical and instrumental analysis techniques. The use of these polymers alone in flame retardant coatings may become expensive while could be made usable by blending with some other conventional polyurethanes6. For this reason conventional castor oil based polyurethane have been selected which is cheap and is used extensively in large numbers of commercial products 7. But this polymer possesses poor mechanical properties due to its high aliphatic nature and is inherently flammable8. Therefore, in the present work, blending of phosphorus based polyurethanes and castor oil based polyurethane have been carried out in different proportions to prepare numbers of formulations which have been used for the coating of metal panels and to prepare casted films. Thermal and flame retardant properties of neat polymers and polymer blends in film form and mechanical properties of the coated films have been found out.
Experimental Sections
Materials
The reagents resorcinol(RC),phenyl dichloro phosphate(PDP),toluene diisocyanate(TDI), isophorone diisocyanate (IPDI), hexamethylene diisocyanate (HMDI), diphenyl methane diisocyanate (MDI), diammonium phosphate((NH₄)₂HPO₄) were obtained from Aldrich and were used without further purification. Castor oil (CO) was obtained from the local market having the hydroxyl value of 153. Solvents methyl ethyl ketone (MEK) and methanol were used after distillation.
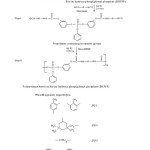
Synthesis of Monomer: Bis-(M-Hydroxy Phenyl) Phenyl Phosphate (BHPPP)
Resorcinol (220 gm, 2.0 mole) and Phenyl dichloro phosphate (195 gm, 1 mole) were reacted in a round bottom flask equipped with reflux condenser at 80 °C under constant stirring over a period of 5.5 hrs in MEK solvent. The content was poured in a calculated amount of ice-cold water and kept overnight for the completion of precipitation. The Product obtained after filtration was crystallized from methanol9. Percentage yield of the monomer is 85%.
Synthesis of Polyurethanes
Polyurethane(PU-1) was synthesized in two steps as shown in reaction scheme 1. In the first step NCO terminated prepolymer was prepared by reacting bis-(m-hydroxyl phenyl) phenyl phosphate (BHPPP, 1.0 mole) with toluene diisocyanate (TDI, 2.0 mole) in a three neck flask at 55 °C with continuous stirring for 3 hours in methyl ethyl ketone (MEK) solvent. In the next step this prepolymer was further reacted with excess monomer (BHPPP) at 80 °C for 2 hours in the same reaction medium to get phosphorous containing polyurethane. The product was vacuum distilled to obtain pure phosphorous containing polyurethane (PU-1).
Other diisocyanates such as isophorone diisocyanate (PU-2), hexamethylene diisocyanate (PU-3) and diphenyl methane diisocyanate (PU-4) were also reacted with BHPPP under the same conditions to obtain the respective polyurethanes.
Synthesis of Castor Oil Based Polyurethane (COPU)
Castor oil (5.0 gm, 0.0057 mole) was allowed to react with toluene diisocyanate (2.088 gm, 0.0121 mole) in a round bottom flask attach with the reflux condenser at 45 °C for 1 hour with continuous stirring. Pure polyurethane was obtained after vacuum distillation11.
Characterization of Monomer and Polymers
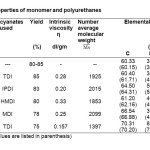
Number of hydroxyl groups of BHPPP was determined by hydroxyl group estimation12. BHPPP contains 1.96 hydroxyl groups per mole of sample. The percentage of free NCO group12 in COPU is 1.5%. Purity of the monomer and polymers was confirmed by thin layer chromatography (TLC). Percentage yield is given in Table 1. Intrinsic viscosity of the polyurethanes was measured using ubbelohde suspended level viscometer in toluene solvent and the values are in between 0.157 to 0.33 dl/gm. The number average molecular weight of COPU as determined by vapour pressure osmometry is 1289. Number average molecular weight of the polyurethanes was determined by gel permeation chromatographic (GPC) technique using Perkin Elmer USA (Model Series 200) and the values are in the range of 1853 to 2099. Elemental analysis of the polyurethanes was carried out in Perkin Elmer USA (Model 2400 series II). Infrared spectrophotometer Perkin Elmer USA (Model Spectrum GX, FT-IR) was used to record the infra-red spectra of the monomer (BHPPP) and polyurethanes.
Curing study was carried out using the differential scanning calorimeter PERKIN ELMER; Model-DSC (Pyris-1).Thermo gravimetric analysis of the cured samples was performed using the thermogravimetric analyzer PERKIN ELMER USA (TGA-7)
Preparation of Films
Two steel plates, free from surface imperfections such as rolling marks, scores, scale etc. having the dimensions 0.1x18x20 cm (Plate A) and 0.3 x 18 x 20 cm (Plate B) were taken. Plate A was cut from inside leaving 2.5 cm edges all around. A silicon-coated paper was placed on the flattened steel plate-B and plate-A was placed above it. The plates were clamped tightly at the edges and sealed with wax. The assembly was placed on a triangular leveling plate10.
Required amount of different phosphorous containing polyurethanes, castor oil based polyurethane, curing agent 0.1 % (NH₄)₂HPO₄ and solvent methyl ethyl ketone (2%) were taken in a beaker and mixed thoroughly and carefully. The homogeneous mixtures thus formed were poured on the prepared site of the casted assembly. The systems were kept for two hours at room temperature and then in a heated oven at require temperature for specific time period. Components used for making films are given in Table 2. The films were post cured at a temperature of 100 0C for 2 hours. The assembly was then cooled to room temperature and declamped. Plate-A was lifted up and the films were peeled off and finally stored in desiccators at room temperature under vacuum. The procedure described above produced films of about 2.11 mm thickness as measured by micrometer screw gauge. Samples of required size were cut for different types of analysis.
Testing of the Films
Flame retardancy of the films was determined by limiting oxygen index (LOI) value (ASTM D2863) and UL-94, vertical burning test method (ASTM D3801). In UL-94 test method sample achieves V-0 rating when it must self extinguished within 10 second and there should not be any sign of dripping the burning polymer. While if the sample continuously burns or if drips the polymer, V-1 or V-2 rating is given13. Smoke density was measured using the test method ASTM D284314. To determine the resistance of the polyurethane films towards UV radiation, the films were exposed to radiation for 3 hours. UV spectra were taken before and after the exposure of the films under UV light. From the spectra and λmax value, UV stability was judged15.
The Universal Testing Machine, SHIMADZU, JAPAN, Model-AUTOGRAPH AG 100 KNG was used for the testing of tensile strength of the films according to ASTM D882 method. To determine the hardness of films, Shore-A hardness tester was used and the tests were carried out according to ASTM D2240 method16.
Preparation of Coatings
Various coating formulations were prepared by mixing different phosphorus based polyurethanes, castor oil based polyurethane, 0.1% (NH₄)₂HPO₄ and methyl ethyl ketone solvent using a magnetic stirrer to get homogeneous solutions. These formulations were applied on mild steel (M.S.) test panels using a film applicator. Care was taken during coating to obtain uniform film thickness of about 100 µm. The coated materials were then kept in an open place at room temperature for specific time period for complete setting10. For proper setting, gel times of the formulations given in Table 5 were used.
Testing of Coatings
Once the coating panels were prepared, next step was the determination of properties. Various properties like hardness, flexibility and adhesion properties were determined by standard methods. The strength of adhesion to the coated panels and flexibility of the polyurethanes and blends were determined respectively by crosshatch and mandrel bend test methods ASTM D3359 and ASTM D522 standards13.Scratch hardness and pencil hardness were measured according to the methods ASTM D2197 and ASTM D3363 respectively11.
Results and Discussion
Microanalysis data of carbon, hydrogen and nitrogen of polyurethanes are in good agreement with the calculated values and are given in Table 1.
The Infrared spectrum of BHPPP monomer shows the broad band around 3350-3400 cm-1 for the hydroxy group stretching frequency. In BHPPP, the absorption band at 981 cm-1 is for P-O-C band and the band at 1230 cm-1 is for P=O group.
The IR spectra of polyurethanes show the absorption bands around 1628-1659 cm-1 for -OCONH asymmetric stretching vibration. The broad bands around 3288-3341 cm-1 are attributed to N-H band. The bands around 1000-1018 cm-1 are for P-O-C band and bands around 1223-1253 cm-1 are for P=O group.
The IR spectrum of COPU shows a band around 3341 cm-1 due to –NH moiety. The weak band at 2929 cm-1 is due to aromatic C-H stretching frequency. Aliphatic symmetric stretching vibrations appear at 2858 cm-1and the bands around 1319-1453 cm-1 are due to asymmetric stretching vibration of aliphatic structure of polyurethane. The sharp band at 2273 cm-1 is due to the presence of –NCO group which is present in all the polyurethanes.
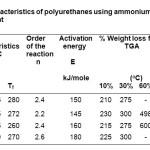
In Table 2, Ti is the initiation of the curing process. Tp is the peak temperature where maximum crosslinking occurs and Tf is final temperature which indicates the completion of the curing process. The data obtained from DSC scans reveal that initiation of curing process for HMDI based system(PU-3) occurs at 202°C while peak temperature(Tp) of this system is 245°C.Ti and Tp of MDI based systems show highest value in the series, followed by TDI based system. The presence of aromatic ring in MDI and TDI lowers the reactivity of these systems. Being cycloaliphatic in the nature, the IPDI based system shows combined behavior of cyclic and aliphatic moiety. Curing characteristics of this system has moderate value, where initiation starts at 220°C and maximum crosslinking occurs at 255°C. The order of the curing processes and activation energy of the systems were obtained using Borchardt and Daniel method. The curing processes follow second order reaction and the activation energy is in the range of 14-180 kJ/mole.
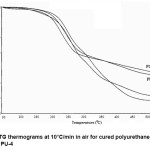
The thermal stability of the cured polyurethanes have been found out using Thermogravimetric analysis.TGA thermograms given in Fig. 2 gives the idea of percentage weight loss data for 10%,30% and 70% weight loss. Table 3 shows that the stability of polyurethanes is in the range of 210-230°C. The systems have moderate stability which is an essential requirement for flame retardant polymers used under this temperature range. During burning polymer starts to decompose and generates phosphoric acids and related compounds resulting in the formation of char layer on the coated films.
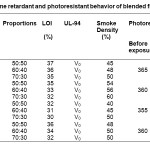
Flame retardant properties like LOI, UL-94 and smoke density are reported in Table 4. As the amount of phosphorus based polyurethanes increases LOI increases. MDI and TDI based Polyurethane systems have higher char value (Table 2) due to their aromatic nature, while HMDI and IPDI based systems give lower char value which is due to their aliphatic characteristics. Thus, PU-1 and PU-4 based blends having 50:50 ratio show LOI value 37% and 36% respectively. All the blends give V0 rating under UL-94 test. Thus the blends can be converted into char very rapidly without melting. Smoke density test shows that the 50:50 proportion of COPU with different PUs have lowest smoke density than the other blends and neat COPU. The polyurethane films were exposed to UV radiation and λmax values before and after exposure show no remarkable change. Thus the polyurethanes can be regarded as UV-resistant.
Table 4 shows the shore hardness properties of the polyurethane films. A decrease in hardness value in case of blends may be due to the flexible nature of castor oil based polyurethane. Tensile strength (Table 4) of the blended films is increased from 30-106% by the incorporation of COPU with respect to the neat polyurethane systems. Elongation at break in these systems also shows a marked increase in the values compare to neat polyurethanes.
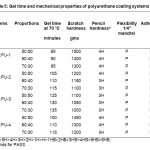
Adhesion of coated panels was tested by crosshatch tester. The films do not remove from the coated panels. Results in Table 5 shows that all the coated panels have 100% adhesion. Flexibility of the coated panels was measured by bending 1/4” mandrel and all the systems pass the mandrel bend test. Scratch hardness(17) of the systems was measured using a mechanically operated scratch hardness tester (Sheen Instrument, U.K.).The scratch hardness of TDI (COPU: PU-1) and MDI (COPU: PU-4) based polyurethane systems have higher scratch resistance than that of the IPDI (COPU: PU-2) and HMDI (COPU: PU-3) based systems due to their aromatic nature. Scratch hardness properties of the blends are in the range of 1000-1500 grams as listed in Table 5. The pencil hardness of all the coated materials also follows the same trend.
Conclusions
Incorporation of phosphorus containing polyurethanes to COPU imparts flame retardancy to COPU. Improvement in tensile strength and elongation is observed in the blended films. The properties of the coated panels in presence of blends are better compare to the neat polyurethanes.
Acknowledgments
The financial assistance from University Grants Commission (UGC), New Delhi, India is gratefully acknowledged. Appreciation is expressed to Sophisticated Instrumentation Center for Applied Research and Testing (SICART), Charutar Vidya Mandal, Vallabh Vidyanagar, for the analysis of the samples.
References
- Datta J. and Balas A. J. Therm. Anal. Calorim., 74: 615–621(2007).
CrossRef
- Chattopadhyay D. K. and Raju K. Prog. Polym. Sci.,32: 352–418 (2007).
CrossRef
- Patel R. H., Patel H. B. and Shah M. D., Int.J.Polym.Anal.Charact.,14: 563-565 (2009).
CrossRef
- Spireckel M., Regnier N., Mortangne B., Yousset B. and Bunel C., Polym. Degrad. Stab., 78: 211-218 (2002).
CrossRef
- Kemmlein S., Hahn O. and Jann O., Atmos.Environ., 37:5485-5493 (2003).
CrossRef
- Yu Lu S. and Hamerton I., Prog.Polym. Sci., 27: 1661-1712 (2002).
CrossRef
- Yeganeh H. and Hojati-Talemi P., Polym. Degrad. Stab. 92: 480-489 (2007).
CrossRef
- Ikeh P.O., Nig. J. Bas. Appl. Sci., 19: 55-63 (2011).
- Wang C. and Shieh J., Eur. Polym. J., 36: 443- 452 (2000).
CrossRef
- Patel R. H. and Patel H. B., Prajna-J. Pure. Appl. Sci., 15: 66-76 (2007).
- Patel R. H., Shah M. D. and Patel H. B., Int. J. Polym. Anal. Chara., 16:107-111 (2011)
CrossRef
- Vogel A. I., Elemental Practical Organic Chemistry.Quantitative Organic Analysi. 2nd ed. CBS Publisher and Distributiors: Delhi, (1987).
- Randall D. and Lee S., The Polyurethane Book. 2nd ed. John Willey: New York, (2002).
- Pal K. and Rastogi J. N., J. Appl. Polym. Sci., 94: 407- 415 (2004).
CrossRef
- Hwang D. K., Moon J. H., Shul Y. G., J. Sol-Gel Sci.Tech. 26: 783-787 (2003).
- Pinto U. A., Visconte L. Y. and Nunes R. C., Eur. Polym. J., 37: 1935-1937 (2001).
CrossRef
- Xiang C., Sui H., Chu J. and Coleman B. J. of Polym. Sci. Part B: Polym. Phy., 39: 47-59 (2001)
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal