Nainsi1 and Nibedita Banik2*
and Nibedita Banik2*
1Master of Chemistry, Chandigarh University, Mohali, Punjab India
2Department of Chemistry, Chandigarh University, Mohali, Punjab India,
corresponding Author E-mail: nibedita.e11769@cumail.in
DOI : http://dx.doi.org/10.13005/msri.20.special-issue1.05
Article Publishing History
Article Received on : 27 Apr 2023
Article Accepted on : 16 Aug 2023
Article Published : 22 Sep 2023
Plagiarism Check: Yes
Reviewed by: Dr. Prashant Koli
Second Review by: Dr. Chandra Mohan
Article Metrics
ABSTRACT:
The most abundant and valuable class of chemicals currently used in industries are referred to as nitroaromatic compounds. These are compounds that include organic molecules and, more importantly, at least one nitro- group in the ring. As we already know, chemistry is currently known for using a variety of fluorescent techniques. Picric acid (O
2N)
3C
6H
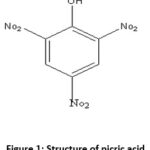
2OH) is an organic compound also known as 2, 4, 6-trinitrophenol (TNP), according to the IUPAC. It is extensively used in the industries of dyes, leather, fireworks, and matches. In this review, author tried to explain how to detect picric acid through fluorescent Chemosensor. It helps in the synthesis of fluorescent Nanoscopic objects or compounds.
KEYWORDS:
Application of Picric Acid; Facts of picric acid; Fluorescent Chemosensor; Harmful effects of picric acid; Picric Acid
Copy the following to cite this article:
Banik N, Nainsi N. Detection of Picric Acid: By Fluorescent Chemosensor (Nitro-Aromatic Compound): A Short Review. Mat. Sci. Res. India; Special Issue (2023).
|
Copy the following to cite this URL:
Banik N, Nainsi N. Detection of Picric Acid: By Fluorescent Chemosensor (Nitro-Aromatic Compound): A Short Review. Mat. Sci. Res. India; Special Issue (2023). Available from: https://bit.ly/48X8Iq1
|
Introduction
The first is that, the nitroaromatic complexes are extremely difficult to find in nature. So, the primary reason for their identification is that we are human 1. Human activities play a significant role in identifying these compounds, and the main thing is that these are explained in nature 2. Nitro- Aromatic compounds played a huge role in an industrial area of chemical sciences due to their explosive nature. These synthesized compounds have a special quality of explosives with huge energy 3. There are so many sensors that we can use but basically, the fluorescent Chemosensor is the best among the various sensors4. The nature of properties of the fluorescent Chemosensor is highly responsible for the research of picric acid 5
Fluorescent chemosensor has an excellent fluorescence intensity at 516nm, making it ideal for profiling anything. The fluorescence sensor possesses high sensitivity toward picric acid. This sensor is specially has been selected for picric acid research 6
We already know that research on nitroaromatic compounds is the most focused topic for chemistry research. Because nitroaromatics are so explosive by nature makes them a interesting research topic 7. And picric acid is one of the main nitroaromatic compounds about their detection by fluorescent Chemosensor, applications which we studied in the following paper8 .Fluorescent Chemosensor is a better sensor than others for detecting the nitroaromatic compound picric acid 9.
Area Covered
We have discussed here
What actually picric acid is
About fluorescent Chemo sensor
Detection of picric acid by fluorescent Chemo sensor
Why it is called as nitro aromatic compound
Applications of picric acid
Harmful effects of picric acid
I used all the literature from different books, different review articles, different research study for this article.
Expert Opinion
Picric acid is an extremely hazardous organic acid. Despite its explosive properties, this acid is a chemical reagent commonly utilized in industrial substances that are extensively utilized in the production of pigments, dyes, insecticides, chemical fibers, medicines, pyrotechnics, and rocket propellant.
As a result, it is critical to develop a simple yet effective method for a highly precise sensation of picric acid in both the vapor and solution phases for the sake of both societal safety and environmental health.
Based on this review study, we can conclude that the identification of this acid will be significant in the future. In this study, the author addressed all of the applications, properties, and harmful effects, as well as all of the basic information. During the process of writing this article, I discovered that a fluorescent Chemosensor is a better sensor than others for detecting the nitroaromatic compound picric acid. This field is still untapped because there are numerous methods for detecting picric acid, including latent fingerprint representation colorimetric, plasmon resonance on the surface, and many others. This field is still untapped because there are numerous methods for detecting picric acid, including latent fingerprint representation colorimetric, plasmon resonance on the surface, and many others. Nonetheless, the aforementioned techniques are not widely available because they require expensive devices and skilled personnel to use.
In the future, research towards detecting picric acid will help in most aspects of lifestyle and the environment, as it is an important factor in the safety of the greenery around us, as well as public health.
Body
Some basic classes of nitroaromatic compounds
Nitrobenzene or we can say oil of mirbane 10
1- methyl-3-nitrobenzene or nitrotoluene 11
Nitrophenols or we can call picric acid 12
Picric acid
The compound is organic and has the formula (O2N)3C6H2OH , and its IUPAC name is TNP13. It is a member of the amalgam which is nitro-aromatic in nature. As the name picric suggests bitter so its name gives the idea of its nature 14. Picric acid is used in the formation of explosives, matches, and electric batteries.15
The very first nitrated organic compound that is explosively involved in military high-ranged weapons for firing or any other work related to it is nitrophenol, also known as picric acid. 16.
Applications of picric acid
Picric acid is commonly used in dyeing, pharmaceutical, and other industries. It has the advantage of being easily soluble in water and capable of contaminating both groundwater and soil 17. Picric acid is also used in the synthesis of some reactions and can be used as a contrasting reagent. It can produce salts when it comes into contact with certain metals 18. This acid is also used in electric batteries. It can also be used for making pf glasses or other materials through glasses. It can also be used in the lab as a chemical agent19.
Facts of picric acid
Now we will discuss Picric acid briefly by stating some basic facts such as
Picric acid is a strong acid and has a properties of blasting explosive.It is harmful to the environment and public safety 20. Picric acid is a much more powerful acid than phenol. The main feature of this acid and the region behind its strongest nature is that it is an electron donor group21. It can break down carbonates and participates in basic titration, which means it is good at titrating with bases.
Another important fact about 2,4-trinitrophenol is that it can be absorbed through the skin or the respiratory tract22. Another fact is that in order to limit dryness, we must seal it in a place that is not hot or cold 23.
The next fact is that in order to prohibit its explosive nature, we must place it in a fully ventilated location because it easily comes into contact with a material that has not been reduced or oxidized24.
Harmful effects of picric acid
Picric acid’s first major harmful hazardous effect is itching in the eyes, which causes vision to become yellowish and the eyes to become riddled with colors25. It also causes a sour throat, headache, diarrhea, and other symptoms.26
The main effect of 2,4-trinitrophenol is that it degrades RBC and harms the kidney27. It has a very negative effect on plants and animals, which means that it is extremely harmful to all living things in an environment. Not only that, but it has a negative impact on our health by eating something related to our consuming items28.
Why do we need to detect this nitroaromatic compound (Picric acid)
As we all know, nitroaromatic compounds (picric acid) have a negative impact on the environment29. Because nitroaromatic compounds are extremely hazardous to the environment. Nitroaromatic compound, including picric acid are known foe hepatotoxic and haematotoxin effect. Picric acid is a strong acid30.Picric acid has the most negative effects compared to any nitroaromatic compound. And we must detect it in order to determine the cause of its explosive nature, because detection necessitates extensive research into specific topics or compounds31.
So, for finding explosiveness or we can say a hazardous thing about this compound firstly we need to detect this compound and the main thing is that it is very difficult to detect this acid that’s 32. And lastly, we know its detection is still observed dare for all researchers who take this topic and I just want to know what the main trouble is which comes in way of its detection33.
The above all are the regions for why we need to detect this nitroaromatic compound or 2,4-trinitrophenol.
Fluorescent Chemosensor
The molecule or compound which builds up a compulsory site or a chromophore and process for connection between any two sites are called a fluorescent chemosensor. This chemosensor field primarily enters the living process after approximately 153 years 31 If it has the ability, we can apply it to various branches of chemistry such as biological science, pharmaceutical sciences, environmental sciences, and so on. Currently, a very broad range of this chemosensor has been established for the research of biological or other field-related species34. There are various kinds of chemosensors, such as absorbance, redox potential, nmr relaxation, and fluorescence. however, in this review we discussed about fluorescent chemosensors35. Fluorescent based sensors are frequently used for the detection of nitroaromatic compounds due to their excellent sensitivity, high precision, and immunity to light scattering.36 They are widely used for ions and neutral biological molecules. in a variety of disciplines, including biology and environmental sciences37.
Detection of Picric Acid Through Fluorescent Chemosensor
There are numerous methods for identifying the majority of the nitroaromatic components in nature. Like here we need to identify the picric acid. So, among those methods, we discussed the identification of picric acid using a fluorescent chemosensor.21.
Because of properties such as explosive nature, the detection of nitroaromatic compounds is critical. Picric acid is also a nitroaromatic compound, and its detection is still a problem38. There are several processes that can be used to detect molecules of picric acid-like gas chromatography resonance-ray diffraction and so on. However, the fluorescent chemosensor is the best technique for detecting it.39.
Now, some examples show how to detect picric acid, such as using an aqueous solution of palladium macrocycle. DCM (CH2Cl2) can be used for extraction in this type of detection40. Through a fluorescence procedure, this sensor pretends to have a good connection with 2,4-trinitrophenol41. Fluorescent polymer is primarily involved in the detection of highly hazardous nitroaromatic compounds, and as we know, picric acid is also included in this nitroaromatic compound, so fluorescent polymer can also be used to detect picric acid42.
Picric acid, as we already know, has the highest dissolving power or solubility, which makes its breakdown much more difficult in the environment, resulting in soil pollution and water pollution that is insufficient for drinking or any other good purpose43. Along with that, the anion of 2,4,6-trinitrophenol is used in areas where wars have occurred, causing soil damage and affecting our daily lives through crops, fruits on soil, and so on 44. As a result, research into nitroaromatic compounds, particularly picric acid, is critical in today’s world, which makes fluorescent chemosensors the best method to identify or research 45.Because this chemosensor seems easy and delicate to detect picric acid and it is advanced in many phases that’s why I have chosen this46.
Understanding the origins of picric acid is advantageous because fluorescent chemosensors have a high ability for detection.47.
The presence of a liquid agent known as DA colouring agent in fluorescent chemosensors makes them ideal for detecting picric acid because this coloring agent has a large dedicating property in the field of detecting various nacs, particularly 2,4,6-trinitrophenol 48 Specifically, the fluorescent chemosensor test paper was designed for the perfect research of picric acid because it provides an advanced understanding of the concept of rapid and widely permitted research of nitroaromatic compounds49. Previously, a fluorescent chemosensor selected for identification of 2,4,6-trinitrophenol was reported, in which the double hydrogen bond with pi-pi interaction in picric acid and fluorescent sensor produces rapid receptor fluorescence and achieves picric acid specific recognition50.
Conclusion
In this paper, author discussed picric acid detection techniques. Picric acid’s harmful effects, as well as its benefits, are discussed. By reading this paper, we can easily conclude that picric acid is directly correlated to nitroaromatic compounds, implying that nitroaromatic compounds have explosive, clustering, and other properties. Not only that, but it concludes that picric acid is the most important nitroaromatic compound that should be detected first. Based on the data presented above, the conclusion is that the fluorescent chemosensor is the best technique for studying picric acid.
Acknowledgement
I am great full to chemistry department of Chandigarh University for giving me this golden chance of writing review paper. I am thankful to all the faculty members of Department of science for the positive and co-operative response with time, energy and valuable suggestions they gave me to fulfil the task. I am thank full to Dr. Nibedita Banik for her valuable guidance and supportive nature.
References
- M. Ahmed, S. Hameed, A. Ihsan, and M. M. Naseer, “Fluorescent thiazol-substituted pyrazoline nanoparticles for sensitive and highly selective sensing of explosive 2,4,6-trinitrophenol in aqueous medium,” Sens Actuators B Chem, vol. 248, pp. 57–62, 2017, doi: 10.1016/j.snb.2017.03.125.
CrossRef - B. Gole, A. K. Bar, and P. S. Mukherjee, “Fluorescent metal-organic framework for selective sensing of nitroaromatic explosives,” Chemical Communications, vol. 47, no. 44, pp. 12137–12139, 2011, doi: 10.1039/c1cc15594f.
CrossRef - Z. Bao et al., “Strongly fluorescent cysteamine-coated copper nanoclusters as a fluorescent probe for determination of picric acid,” Microchimica Acta, vol. 185, no. 11, Nov. 2018, doi: 10.1007/s00604-018-3049-2.
CrossRef - V. Sathish, A. Ramdass, M. Velayudham, K. L. Lu, P. Thanasekaran, and S. Rajagopal, “Development of luminescent sensors based on transition metal complexes for the detection of nitroexplosives,” Dalton Transactions, vol. 46, no. 48. Royal Society of Chemistry, pp. 16738–16769, 2017. doi: 10.1039/c7dt02790g.
CrossRef - P. L. Le Barny et al., “Detection of nitroaromatic compounds based on photoluminescent side chain polymers,” in Optically Based Biological and Chemical Sensing, and Optically Based Materials for Defence, SPIE, Oct. 2005, p. 59900S. doi: 10.1117/12.631084.
CrossRef - W. Shu, C. Guan, W. Guo, C. Wang, and Y. Shen, “Conjugated poly(aryleneethynylenesiloles) and their application in detecting explosives,” J Mater Chem, vol. 22, no. 7, pp. 3075–3081, Feb. 2012, doi: 10.1039/c1jm15535k.
CrossRef - K. Dhanunjayarao, V. Mukundam, and K. Venkatasubbaiah, “Tetracoordinate Imidazole-Based Boron Complexes for the Selective Detection of Picric Acid,” Inorg Chem, vol. 55, no. 21, pp. 11153–11159, Nov. 2016, doi: 10.1021/acs.inorgchem.6b01767.
CrossRef - G. V Zyryanov, D. S. Kopchuk, I. S. Kovalev, E. V Nosova, V. L. Rusinov, and O. N. Chupakhin, “Chemosensors for detection of nitroaromatic compounds (explosives),” Russian Chemical Reviews, vol. 83, no. 9, pp. 783–819, Sep. 2014, doi: 10.1070/rc2014v083n09abeh004467.
CrossRef - K. Shen, Z. Ju, L. Qin, T. Wang, and H. Zheng, “Two stable 3D porous metal-organic frameworks with high selectivity for detection of PA and metal ions,” Dyes and Pigments, vol. 136, pp. 515–521, Jan. 2017, doi: 10.1016/j.dyepig.2016.09.011.
CrossRef - D. Wu, A. C. Sedgwick, T. Gunnlaugsson, E. U. Akkaya, J. Yoon, and T. D. James, “Fluorescent chemosensors: The past, present and future,” Chem Soc Rev, vol. 46, no. 23, pp. 7105–7123, 2017, doi: 10.1039/c7cs00240h.
CrossRef - A. S. Tanwar, L. R. Adil, M. A. Afroz, and P. K. Iyer, “Inner Filter Effect and Resonance Energy Transfer Based Attogram Level Detection of Nitroexplosive Picric Acid Using Dual Emitting Cationic Conjugated Polyfluorene,” ACS Sens, vol. 3, no. 8, pp. 1451–1461, Aug. 2018, doi: 10.1021/acssensors.8b00093.
CrossRef - M. Serhan et al., “Total iron measurement in human serum with a smartphone,” in AIChE Annual Meeting, Conference Proceedings, American Institute of Chemical Engineers, 2019. doi: 10.1039/x0xx00000x.
- X. Qi, Y. Jin, N. Li, Z. Wang, K. Wang, and Q. Zhang, “A luminescent heterometallic metal-organic framework for the naked-eye discrimination of nitroaromatic explosives,” Chemical Communications, vol. 53, no. 74, pp. 10318–10321, 2017, doi: 10.1039/c7cc05345b.
CrossRef - K. K. Kartha, A. Sandeep, V. K. Praveen, and A. Ajayaghosh, “Detection of nitroaromatic explosives with fluorescent molecular assemblies and π – Gels,” Chemical Record, vol. 15, no. 1, pp. 252–262, 2015, doi: 10.1002/tcr.201402063.
CrossRef - G. V Zyryanov, D. S. Kopchuk, I. S. Kovalev, E. V Nosova, V. L. Rusinov, and O. N. Chupakhin, “Chemosensors for detection of nitroaromatic compounds (explosives),” Russian Chemical Reviews, vol. 83, no. 9, pp. 783–819, Sep. 2014, doi: 10.1070/rc2014v083n09abeh004467.
CrossRef - B. Roy, A. K. Bar, B. Gole, and P. S. Mukherjee, “Fluorescent tris-imidazolium sensors for picric acid explosive,” Journal of Organic Chemistry, vol. 78, no. 3, pp. 1306–1310, 2013, doi: 10.1021/jo302585a.
CrossRef - B. K. Kundu, Pragti, Reena, S. M. Mobin, and S. Mukhopadhyay, “Mechanistic and thermodynamic aspects of a pyrene-based fluorescent probe to detect picric acid,” New Journal of Chemistry, vol. 43, no. 29, pp. 11483–11492, 2019, doi: 10.1039/c9nj02342a.
CrossRef - M. Ansari, R. Bera, S. Mondal, and N. Das, “Triptycene-Derived Photoresponsive Fluorescent Azo-Polymer as Chemosensor for Picric Acid Detection,” ACS Omega, vol. 4, no. 5, pp. 9383–9392, 2019, doi: 10.1021/acsomega.9b00497.
CrossRef - P. Madhu and P. Sivakumar, “Curcumin-based fluorescent chemosensor for selective and efficient detection of picric acid,” J Mol Struct, vol. 1185, pp. 410–415, 2019, doi: 10.1016/j.molstruc.2019.02.112.
CrossRef - J. R. Zhang, Y. Y. Yue, H. Q. Luo, and N. B. Li, “Supersensitive and selective detection of picric acid explosive by fluorescent Ag nanoclusters,” Analyst, vol. 141, no. 3, pp. 1091–1097, 2016, doi: 10.1039/c5an02251g.
CrossRef - V. Venkatramaiah, S. Kumar, and S. Patil, “Fluoranthene based fluorescent chemosensors for detection of explosive nitroaromatics,” Chemical Communications, vol. 48, no. 41, pp. 5007–5009, 2012, doi: 10.1039/c2cc31606d.
CrossRef - B. Roy, A. K. Bar, B. Gole, and P. S. Mukherjee, “Fluorescent tris-imidazolium sensors for picric acid explosive,” Journal of Organic Chemistry, vol. 78, no. 3, pp. 1306–1310, 2013, doi: 10.1021/jo302585a.
CrossRef - R. Sun, X. Huo, H. Lu, S. Feng, D. Wang, and H. Liu, “Recyclable fluorescent paper sensor for visual detection of nitroaromatic explosives,” Sens Actuators B Chem, vol. 265, pp. 476–487, 2018, doi: 10.1016/j.snb.2018.03.072.
CrossRef - A. Das, S. Jana, and A. Ghosh, “Modulation of Nuclearity by Zn(II) and Cd(II) in Their Complexes with a Polytopic Mannich Base Ligand: A Turn-On Luminescence Sensor for Zn(II) and Detection of Nitroaromatic Explosives by Zn(II) Complexes,” Cryst Growth Des, vol. 18, no. 4, pp. 2335–2348, 2018, doi: 10.1021/acs.cgd.7b01752.
CrossRef - J. Rocha, L. D. Carlos, F. A. A. Paz, and D. Ananias, “Luminescent multifunctional lanthanides-based metal–organic frameworks,” Chem Soc Rev, vol. 40, no. 2, pp. 926–940, 2011, doi: 10.1039/c0cs00130a.
CrossRef - X. Tian, X. Qi, X. Liu, and Q. Zhang, “Selective detection of picric acid by a fluorescent ionic liquid chemosensor,” Sens Actuators B Chem, vol. 229, pp. 520–527, Jun. 2016, doi: 10.1016/j.snb.2016.02.016.
CrossRef - A. Kathiravan et al., “Pyrene-Based Chemosensor for Picric Acid – Fundamentals to Smartphone Device Design,” Anal Chem, vol. 91, no. 20, pp. 13244–13250, 2019, doi: 10.1021/acs.analchem.9b03695.
CrossRef - A. Mishra, R. Dheepika, P. A. Parvathy, P. M. Imran, N. S. P. Bhuvanesh, and S. Nagarajan, “Fluorescence quenching based detection of nitroaromatics using luminescent triphenylamine carboxylic acids,” Sci Rep, vol. 11, no. 1, pp. 1–10, 2021, doi: 10.1038/s41598-021-97832-0.
CrossRef - A. Hakimifar and A. Morsali, “High-sensitivity detection of nitroaromatic compounds (NACs) by the pillared-layer metal-organic framework synthesized via ultrasonic method,” Ultrason Sonochem, vol. 52, pp. 62–68, 2019, doi: 10.1016/j.ultsonch.2018.11.002.
CrossRef - P. A. Parvathy, R. Dheepika, R. Abhijnakrishna, P. K. M. Imran, and S. Nagarajan, “Fluorescence quenching of triarylamine functionalized phenanthroline-based probe for detection of picric acid,” J Photochem Photobiol A Chem, vol. 401, Oct. 2020, doi: 10.1016/j.jphotochem.2020.112780.
CrossRef - N. Venkatramaiah, S. Kumar, and S. Patil, “Femtogram detection of explosive nitroaromatics: Fluoranthene-based fluorescent chemosensors,” Chemistry – A European Journal, vol. 18, no. 46, pp. 14745–14751, 2012, doi: 10.1002/chem.201201764.
CrossRef - S. Kaja, D. P. Damera, and A. Nag, “A metal-enhanced fluorescence sensing platform for selective detection of picric acid in aqueous medium,” Anal Chim Acta, vol. 1129, pp. 12–23, 2020, doi: 10.1016/j.aca.2020.07.001.
CrossRef - A. H. Malik, S. Hussain, A. Kalita, and P. K. Iyer, “Conjugated polymer nanoparticles for the amplified detection of nitro-explosive picric acid on multiple platforms,” ACS Appl Mater Interfaces, vol. 7, no. 48, pp. 26968–26976, 2015, doi: 10.1021/acsami.5b08068.
CrossRef - J. Y. Lee et al., “Ratiometric Turn-On Fluorophore Displacement Ensembles for Nitroaromatic Explosives Detection,” J Am Chem Soc, vol. 142, no. 46, pp. 19579–19587, 2020, doi: 10.1021/jacs.0c08106.
CrossRef - K.-S. Ju and R. E. Parales, “Nitroaromatic Compounds, from Synthesis to Biodegradation,” Microbiology and Molecular Biology Reviews, vol. 74, no. 2, pp. 250–272, Jun. 2010, doi: 10.1128/mmbr.00006-10.
CrossRef - M. Fabin, M. Łapkowski, and T. Jarosz, “Methods for Detecting Picric Acid—A Review of Recent Progress,” Applied Sciences (Switzerland), vol. 13, no. 6. MDPI, Mar. 01, 2023. doi: 10.3390/app13063991.
CrossRef - V. Kumar, N. Choudhury, A. Kumar, P. De, and S. Satapathi, “Poly-tryptophan/carbazole based FRET-system for sensitive detection of nitroaromatic explosives,” Opt Mater (Amst), vol. 100, Feb. 2020, doi: 10.1016/j.optmat.2020.109710.
CrossRef - Y. Peng, A. J. Zhang, M. Dong, and Y. W. Wang, “A colorimetric and fluorescent chemosensor for the detection of an explosive-2,4,6-trinitrophenol (TNP),” Chemical Communications, vol. 47, no. 15, pp. 4505–4507, 2011, doi: 10.1039/c1cc10400d.
CrossRef - Z. Hu, B. J. Deibert, and J. Li, “Luminescent metal-organic frameworks for chemical sensing and explosive detection,” Chem Soc Rev, vol. 43, no. 16, pp. 5815–5840, 2014, doi: 10.1039/c4cs00010b.
CrossRef - Y. Zhao, L. Xu, F. Kong, and L. Yu, “Design and preparation of poly(tannic acid) nanoparticles with intrinsic fluorescence: A sensitive detector of picric acid,” Chemical Engineering Journal, vol. 416, no. February, p. 129090, 2021, doi: 10.1016/j.cej.2021.129090.
CrossRef - K. Acharyya and P. S. Mukherjee, “A fluorescent organic cage for picric acid detection,” Chemical Communications, vol. 50, no. 99, pp. 15788–15791, 2014, doi: 10.1039/c4cc06225f.
CrossRef - P. Jana, M. Yadav, T. Kumar, and S. Kanvah, “Benzimidazole-acrylonitriles as chemosensors for picric acid detection,” J Photochem Photobiol A Chem, vol. 404, no. August 2020, p. 112874, 2021, doi: 10.1016/j.jphotochem.2020.112874.
CrossRef - S. Chakravarty, B. Gogoi, and N. Sen Sarma, “Fluorescent probes for detection of picric acid explosive: A greener approach,” J Lumin, vol. 165, pp. 6–14, 2015, doi: 10.1016/j.jlumin.2015.04.006.
CrossRef - Y. Zhang, B. Li, H. Ma, L. Zhang, and W. Zhang, “An RGH-MOF as a naked eye colorimetric fluorescent sensor for picric acid recognition,” J Mater Chem C Mater, vol. 5, no. 19, pp. 4661–4669, 2017, doi: 10.1039/c7tc00936d.
CrossRef - S. K. Nandi, S. Roy Chowdhury, D. Podder, P. K. Ghorai, and D. Haldar, “A Robust Tripeptide for In-Field Selective Naked Eye Ultratrace Detection of 2,4,6-Trinitrophenol,” Cryst Growth Des, vol. 20, no. 3, pp. 1884–1890, Mar. 2020, doi: 10.1021/acs.cgd.9b01591.
CrossRef - S. Kumar, R. Kishan, P. Kumar, S. Pachisia, and R. Gupta, “Size-Selective Detection of Picric Acid by Fluorescent Palladium Macrocycles,” Inorg Chem, vol. 57, no. 4, pp. 1693–1697, 2018, doi: 10.1021/acs.inorgchem.7b02813.
CrossRef - X. Sun, X. Ma, C. V. Kumar, and Y. Lei, “Protein-based sensitive, selective and rapid fluorescence detection of picric acid in aqueous media,” Analytical Methods, vol. 6, no. 21, pp. 8464–8468, 2014, doi: 10.1039/c4ay01941e.
CrossRef - Z. J. Wang, L. Qin, J. X. Chen, and H. G. Zheng, “H-Bonding Interactions Induced Two Isostructural Cd(II) Metal-Organic Frameworks Showing Different Selective Detection of Nitroaromatic Explosives,” Inorg Chem, vol. 55, no. 21, pp. 10999–11005, 2016, doi: 10.1021/acs.inorgchem.6b01521.
CrossRef - R. Rajak, M. Saraf, S. K. Verma, R. Kumar, and S. M. Mobin, “Dy(III)-Based Metal-Organic Framework as a Fluorescent Probe for Highly Selective Detection of Picric Acid in Aqueous Medium,” Inorg Chem, vol. 58, no. 23, pp. 16065–16074, 2019, doi: 10.1021/acs.inorgchem.9b02611.
CrossRef - C. Zhou et al., “A Fluorescent Chemosensor with a Hybridized Local and Charge Transfer Nature and Aggregation-Induced Emission Effect for the Detection of Picric Acid,” ChemistrySelect, vol. 4, no. 10, pp. 2868–2873, 2019, doi: 10.1002/slct.201900294.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.
 and Nibedita Banik2*
and Nibedita Banik2*
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal