Ayse Kurt1,2 and Ayse Kalemtas1*
and Ayse Kalemtas1*
1Bursa Technical University, Faculty of Engineering and Natural Sciences, Department of Metallurgical and Materials Engineering, Bursa, Türkiye
2BUTEKOM, Bursa, Türkiye
Corresponding Author E-mail: ayse.kalemtas@btu.edu.tr
DOI : http://dx.doi.org/10.13005/msri/200302
Article Publishing History
Article Received on : 07 Dec 23
Article Accepted on : 27 Jan 2024
Article Published : 27 Jan 2024
Plagiarism Check: Yes
Reviewed by: Dr. Masoud Taghavi Second Review by: Dr. Ilyes Benharrath
Second Review by: Dr. Ilyes Benharrath Final Approval by: Dr. Sadek Khalifa Mohammed Shakshooki
Final Approval by: Dr. Sadek Khalifa Mohammed Shakshooki
Article Metrics
ABSTRACT:
In this study, porous zeolite spheres were produced at a low temperature by a facile and economical method, sol-gel, using a natural zeolite from the Gördes region of Türkiye and waste soda glass powder. Waste glass powder was achieved by milling the recyclable waste soda glass bottles to be used as a source of silica. Elemental analysis of the waste glass was carried out by using X-ray fluorescence spectrometry. It was determined that Si (57.3 wt. %), Ca (20.9 wt. %), Na (13.9 wt. %), Mg (2.64 wt. %), and Al (1.64 wt. %) were the major constituents of the waste glass. Three different sphere compositions were designed containing 1:1, 3:2, and 7:3 zeolite:waste glass ratio. When the zeolite:waste glass ratio was 1:1 oval-shaped green spheres were achieved. For the compositions containing 3:2, and 7:3 zeolite:waste glass ratio spherical green samples were achieved. Prepared spheres were sintered at 300°, 400°, and 500°C for 1 h. It was observed that the samples could not maintain their spherical form when the sintering temperature was lower than 500°C. Scanning electron microscopy investigation of the spheres sintered at 500°C revealed that highly porous zeolite spheres, containing pores from ~20 µm to nanometre sizes, were achieved. Image J software was used to determine effect of composition on the size and size distribution of the sintered spheres.
KEYWORDS:
Image J; Low temperature sintering; porous ceramic spheres; sol-gel; waste glass; Zeolite
Copy the following to cite this article:
Kurt A, Kalemtas A. Low Temperature Sintering of Porous Zeolite Spheres via Waste Glass Powder Addition. Mat. Sci. Res. India; Special Issue (2023).
|
Copy the following to cite this URL:
Kurt A, Kalemtas A. Low Temperature Sintering of Porous Zeolite Spheres via Waste Glass Powder Addition. Mat. Sci. Res. India; Special Issue (2023). Available from: https://bit.ly/3HxUhwL
|
Introduction
Porous ceramics have a wide range of industrial applications, such as filtration, absorption, thermal insulation, scaffolds, and catalyst supports with high specific strength, low density, and excellent thermal properties 1. The production method for the porous ceramic materials varies according to the desired pore size and distribution. Partial sintering, sacrificial templating, direct foaming, and the use of pore-forming agents are the standard processing techniques that can be used to produce porous ceramics 2,3.
Achieving a desired specific pore size that can be controlled is essential for producing adsorbents. Besides managing the size of the pore, chemical groups on the adsorbents significantly affect the filtration process. Due to strong infrared active vibration groups, ceramic materials can change water’s physical and chemical properties. Zeolite is one of the most widely used materials to remove impurities in water.
Zeolites are aluminosilicate minerals containing rich alkali and alkaline earth elements, which can be produced synthetically and occur naturally in volcanic regions. More than 40 natural zeolite minerals were discovered, and the most common zeolite minerals are clinoptilolite, heulandite, and chabazite. Also, more than 150 zeolites have been synthesized 4. Zeolites are minerals with a three-dimensional structure, which have aluminum, silicon, and oxygen in their lattice structures, channels containing cations and water in their pores, and interconnected voids 5. Since zeolites are porous materials, they are very advantageous for providing porosity. Zeolites can selectively remove heavy metals such as lead and cadmium and specific cations such as ammonia. It also has an excellent ion exchangeability to adsorb various ionic substances 6. Due to its unique advantages, zeolites has massive potential in filtration and purification technologies and membrane reactor applications. It is known that natural zeolites have significant potential in water and wastewater treatment, especially in hardness removal and heavy metal removal. It is also used in the energy, agriculture, livestock, and health sectors 7.
The most important and well-known deposits of clinoptilolite, one of the most common zeolites in Türkiye, are in Gördes and Bigadiç regions. Clinoptilolite reserves are estimated to be 2 billion tons in the Manisa Gördes field, the only zeolite reserve licensed by the General Directorate of Mineral Research and Exploration of Türkiye 8. It is reported in the literature that Gördes zeolite is an ion exchanger suitable for removing heavy metals and controlling environmental pollution. It has approximately 9 % more ammonia removal capacity than Bigadiç zeolite 9,10.
In recent years, the focus of investigation on water filtration systems has been on the development of simple, economical, efficient, and innovative methods. In the literature, there are many studies on wastewater treatment; however, there are limited studies on obtaining potable water from seawater or achieving tap water. In this context, there is a need to develop new methods. Currently, using porous ceramic spheres for water filtration is a unique system. Many methods are used to produce porous ceramic adsorbents, but the use of toxic substances or high sintering temperatures prevents their use 11. Lowering the process temperature without decreasing the mechanical strength is necessary to develop low-cost ceramic adsorbents.
In the literature, the sintering temperature is reported as 800-1350°C in studies related to the production of zeolite-based porous ceramics 12,14. Previous studies conclude that increasing the sintering temperature increases mechanical strength. In contrast, it decreases the water absorbability due to increased pore size, decreased porosity, and increased crystallinity of the glass phase 14. A recent study reported developing zeolite-based porous ceramic using economic natural zeolite powder with Bi2O3, B2O3, and SiO2 additives and sintered at relatively low temperatures (500-600 °C) 14. In this study, a novel approach was applied to produce porous zeolite spheres. To the best of our knowledge, a study on the use of waste galss as a source of sintering additive in zeolite-based porous ceramic spheres is not available in the literature.
The aim of the current study is to produce porous ceramic spheres at low temperatures with natural and local raw materials suitable for recycling. Ceramic sphere production was carried out by using sodium alginate with the sol-gel method, which is economical and easily applicable. The effects of sintering conditions and chemical composition on the pore size and distribution of the developed porous ceramic spheres were characterized.
Material And Method
In this study, zeolite supplied from Gördes Zeolite Mining Inc., Türkiye and waste glass powder achieved via milling waste soda glass bottles were used as starting raw materials. The obtained waste glass powder was used as a sintering additive. Elemental analyses of the starting raw materials were carried out by the Rigaku-Supermini model XRF device. In order to obtain glass powder from waste soda glass bottles, they were washed and dried in an oven. Then, they are crushed into coarse aggregate sizes and ground in a vibratory disc mill to achieve waste glass powder. Porous ceramic sphere production was carried out by applying the sol-gel method using sodium alginate. Initially, a homogeneous solution containing 2 wt. % sodium alginate (Katki Dünyasi, Türkiye) was prepared by mixing in a magnetic stirrer for 24 hours. Then, 150 ml of sodium alginate solution, zeolite, and waste glass powder were milled by planetary ball milling at 350 rpm for 30 minutes to obtain a homogeneous ceramic slurry. Three compositions with different zeolite/waste glass powder ratios were designed, as given in Table 1. As a deflocculant, Dolapix CE 64 (1 wt. %) was used to adjust the rheological properties of the slurry.
Table 1: Designed compositions for the production of porous ceramic spheres.
|
Composition
|
Zeolite (wt. %)
|
Waste glass powder (wt. %)
|
|
Z50C50
|
50
|
50
|
|
Z60C40
|
60
|
40
|
|
Z70C30
|
70
|
30
|
In addition, an aqueous solution containing 1 wt. % calcium chloride (Katki Dünyasi, CaCl2) to be used in the crosslinking process in the ceramic sphere production process was prepared by magnetic stirring for 1 hour at room temperature. Then, the prepared ceramic slurry was added dropwise to the CaCl2 aqueous solution using a 60 ml hypodermic syringe through a needle under constant stirring at room temperature. Figure 1 shows a photograph of the green sphere samples that were produced.
The produced spheres were then sintered at 300°C, 400°C, and 500°C for 1 hour under atmospheric conditions. The heating and cooling rates were set at 10°C/min. Microstructural development of the sintered sphere samples were examined by using Carl Zeiss/Gemini 300 model SEM device operating at an accelerating voltage of 15 kV.
Results and Discussion
The chemical analysis of the starting raw materials used in this study is given in Table 2. The main element of the zeolite mineral is silicon, and also it contains a high amount of aluminium and potassium. In addition to a significant amount of iron, calcium, and magnesium elements, it has also been observed that zeolite contains various elements (such as sodium, manganese, titanium, etc.), each below 0.5%. These results are consistent with those reported in the literature [8,15]. Clinoptilolite, a natural zeolite, contains high amounts of potassium and calcium and low amounts of magnesium and sodium [8]. However, the composition, purity, and mineralogical properties of clinoptilolite can vary widely from one deposit to another and even within the same deposit. Iron content is associated with impurities such as iron oxide in zeolitic tuffs [8]. Natural zeolite is close to the composition of glasses containing alkali and alkaline earth metal cations [16]. XRF results revealed that the main element in the glass powder obtained from waste soda glass bottles is silicon, which also contains high amounts of calcium and sodium.
Table 2: XRF results of zeolite and waste glass powder
|
Element
|
Zeolite (wt. %)
|
Waste glass powder (wt. %)
|
|
Si
|
63.300
|
57.300
|
|
Al
|
11.200
|
1.640
|
|
K
|
10.900
|
0.695
|
|
Fe
|
5.890
|
0.836
|
|
Ca
|
5.880
|
20.900
|
|
Mg
|
0.812
|
2.640
|
|
Na
|
0.537
|
13.900
|
|
Rh
|
0.507
|
–
|
|
Ti
|
0.286
|
0.160
|
|
Mn
|
0.236
|
–
|
|
Cr
|
–
|
0.923
|
|
W
|
–
|
0.602
|
|
S
|
–
|
0.232
|
Figure 2 shows the image of Z50C50 samples sintered at three different temperatures. When the macroscopic images of the spheres are examined in Figure 2, it is observed that the spheres are reduced in size, and the samples are in different colors depending on the applied sintering temperatures. It was reported that the dehydration of the zeolite and the recrystallization process in which some physicochemical reactions can change the microstructure may cause a color change in the sample [12]. Samples could not maintain their spherical form when the sintering temperature was 300°C and 400°C. In addition, it was observed that different structures were formed on the outer surface of the spheres. This was attributed to incomplete phase transitions. For this reason, sintering studies for the other compositions were continued at 500°C.
The images of the spheres produced in different compositions, sintered at 500°C for 1 hour, are given in Figure 3. Spherical products were obtained for Z60C40 and Z70C30 samples. However, a few oval-shaped products were also observed for the Z60C40 sample. Image J software was used to investigate the size and size distribution of the spheres depending on the composition. Image processing was carried out with 50 randomly selected spheres from each composition. Corresponding histograms of the images are given in Figure 4. It was determined that 68 % of the spheres were 1.754- 2.034 mm, and 26 % had a diameter of more than 2 mm for Z50C50. For Z60C40, it was determined that the size of the spheres is less than 2 mm, and 72 % of the spheres are in the size range of 1.372-1.564 mm. For Z70C30, 32 % of the spheres had a diameter of more than 2 mm, and 80 % were in the size range of 1.783-2.083 mm.
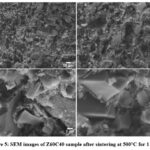
SEM images of fracture surfaces of the Z60C40 sample are given in Figure 5. These images revealed that the pore size distribution of the produced sample is changing from ~20 µm to nanometre sizes. This wide range porosity was attributed to the formation of the liquid phase with the addition of waste glass powder as a sintering additive. In previous studies, the decrease in density was explained by the increase in the content of the glassy phase, which exhibits low density or high porosity [12,17].
Conclusion
In this study, the production of porous spherical zeolite ceramics in millimeter sizes was successfully achieved using environmentally friendly starting raw materials, zeolite, and waste glass powder at a relatively low sintering temperature.
Waste glass powder elemental analysis was performed by X-ray fluorescence spectrometry. It was determined that Si (57.3 wt. %), Ca (20.9 wt. %), Na (13.9 wt. %), Mg (2.64 wt. %), and Al (1.64 wt. %) were the major constituents of the waste glass.
Porous zeolite-based ceramic spheres were shaped by the sol-gel method, which is an easy, relatively rapid, and economical method to produce ceramic-polymer-containing spheres.
Three different spheres containing 1:1, 3:2, and 7:3 zeolite:waste glass ratios were designed. When the zeolite:waste glass ratio was 1:1, oval-shaped green spheres were achieved. Spherical green samples were achieved for the compositions containing 3:2, and 7:3 zeolite:waste glass ratio.
Sintering studies were carried out at 300°, 400°, and 500°C for 1 h. It was noticed that the samples could not maintain their spherical geometry when the sintering temperatures were 300° and 400°C.
Scanning electron microscopy investigation of the spheres sintered at 500°C revealed that highly porous zeolite spheres, containing pores from ~20 µm to nanometre sizes, were achieved.
The size of the spheres sintered at 500°C was investigated with Image J software. It was determined that 68 % of the spheres were in the size range of 1.75- 2.03 mm, and 26 % had a diameter of more than 2 mm for the composition Z50C50. For Z60C40 composition, sphere sizes were less than 2 mm, and 72 % were in the size range of 1.37-1.56 mm. For the Z70C30 composition, 32 % of the spheres had a diameter of more than 2 mm, and 80 % were in the size range of 1.8-2.08 mm.
To the best of our knowledge, a study on the use of waste glass as a source of sintering additive in zeolite-based porous ceramic spheres is not available in the literature. Produced porous zeolite based ceramic spheres may have the potential to be used directly or as a reinforcement for a composite material production that can be used in various applications, such as liquid or gas filtration applications.
Acknowledgments
The authors gratefully acknowledge to Bursa Technical University, Scientific Research Project Unit.
Conflict of Interest
There are no conflict of interest
Funding source
Bursa Technical University, Scientific Research Project Unit (Project No: 220T016).
References
- Ohji T., Fukushima M. Macro-porous ceramics: processing and properties. International Materials Reviews. 2012;57(2):115-131.
CrossRef - Al-Naib U. M. B. Introductory chapter: a brief introduction to porous ceramic. In Recent advances in porous ceramics. IntechOpen. 2018.
CrossRef - Kalemtaş A. Gözenekli Kordiyerit Küre Üretim ve Karakterizasyonu. Afyon Kocatepe Üniversitesi Fen ve Mühendislik Bilimleri Dergisi. 2019:376-384.
- Stocker K., Ellersdorfer M., Lehner M., Raith J.G. Characterization and utilization of natural zeolites in technical applications. BHM Berg-und Hüttenmännische Monatshefte. 2017;162(4):142-147.
CrossRef - Demirkiran A. Ş., Artir R., Avcı E. Effect of natural zeolite addition on sintering kinetics of porcelain bodies. Journal of Materials Processing Technology. 2008;203(1-3):465-470.
CrossRef - Gao N. F., Kume S., Watari K. Zeolite–carbon composites prepared from industrial wastes:(I) Effects of processing parameters. Materials Science and Engineering: A. 2005;399(1-2):216-221.
CrossRef - Othman M. D., Adam M. R., Hubadillah S. K., Puteh M. H., Harun Z., Ismail A. F. Evaluating the sintering temperature control towards the adsorptivity of ammonia onto the natural zeolite based hollow fibre ceramic membrane. International Journal of Engineering. 2018;31(8):1398-1405.
CrossRef - Alver B., Sakizci M., Yörükoğullari E. Investigation of clinoptilolite rich natural zeolites from Turkey: a combined XRF, TG/DTG, DTA and DSC study. Journal of Thermal Analysis and Calorimetry. 2010;100(1):19-26.
CrossRef - Oter O., Akcay H. Use of natural clinoptilolite to improve water quality: sorption and selectivity studies of lead (II), copper (II), zinc (II), and nickel (II). Water Environment Research. 2007;79(3):329-335.
CrossRef - Ugurlu A., Pinar A. Doğal zeolitlerin atıksu arıtımında kullanımı. Jeoloji Mühendisliği Dergisi. 2004;28(2):13-20.
- Klein T. Y., Treccani L., Rezwan K. Ceramic microbeads as adsorbents for purification technologies with high specific surface area, adjustable pore size, and morphology obtained by ionotropic gelation. Journal of the American Ceramic Society. 2012;95(3):907-914.
CrossRef - Ibrahim J. E. F., Kurovics E., Tihtih M., Gömze L. A. Ceramic bricks with enhanced thermal insulation produced from natural zeolite. Pollack Periodica, 2021;16(3):101-107.
CrossRef - Şan O., Abalı S., Hoşten Ç. Fabrication of microporous ceramics from ceramic powders of quartz–natural zeolite mixtures. Ceramics international. 2003;29(8):927-931.
CrossRef - Rajpoot S., Kang E. S., Kim Y. W. Processing and properties of water-absorbing zeolite-based porous ceramics. Journal of the Korean Ceramic Society. 2022:1-10.
CrossRef - Terzi M., Özdemir O. Effects of Hydrochloric and Acetic Acid Modification on the HTAB and K+ Adsorption Characteristics of Natural Zeolites. Dokuz Eylül Üniversitesi Mühendislik Fakültesi Fen ve Mühendislik Dergisi. 2021;23(69):1049-1056.
CrossRef - Vereshagin V. I., Sokolova S. N. Granulated foam glass–ceramic material from zeolitic rocks. Construction and Building Materials. 2008;22(5):999-1003.
CrossRef - Tunç T., Demirkıran A. Ş. The effects of mechanical activation on the sintering and microstructural properties of cordierite produced from natural zeolite. Powder Technology. 2014;260:7-14.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.
 and Ayse Kalemtas1*
and Ayse Kalemtas1*


 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal