S. Sangeethavanathi 1, 2* , P. Gowthaman1
, P. Gowthaman1 , S. Vigneswaran2
, S. Vigneswaran2 and M. Sathishkumar3
and M. Sathishkumar3
1Department of Electronics, Erode Arts and Science College, Erode, India.
2Department of Electronics, Sri Vasavi College, Erode, India.
3Department of Electronics, Nehru Arts and Science College, Coimbatore, India.
Corresponding Author E-mail: rameshvanathi@gmail.com
Article Publishing History
Article Received on : 04 Apr 2024
Article Accepted on : 15 May 2024
Article Published : 22 May 2024
Plagiarism Check: Yes
Reviewed by: Dr. Dipayan Pal Second Review by: Dr. Anil Khairnar
Second Review by: Dr. Anil Khairnar Final Approval by: Dr. K M Garadkar
Final Approval by: Dr. K M Garadkar
Article Metrics
ABSTRACT:
This study delves into the development and characterization of MoS
2 nanoparticles, employing a hydrothermal approach. The synthesized MoS
2 nanoparticles underwent comprehensive analysis utilizing various analytical techniques such as X-ray Diffraction (XRD), Raman spectroscopy, Field Emission Scanning Electron Microscopy (FESEM), Energy Dispersive X-ray spectroscopy (EDX), UV-Visible spectroscopy, and BET surface analysis. XRD analysis revealed the presence of a hexagonal phase structure with a crystallite size of 13 nm, indicating the nanoscale nature of the synthesized material. RAMAN spectroscopy confirmed the presence of characteristic peaks corresponding to Mo and S, validating the composition of the composites. FESEM images shows that the formation of flake like morphology and EDX affirmed the presence of Mo and S elements with the absence of other impurities, ensuring the purity of the MoS
2 nanoparticles. UV-visible spectroscopy exhibited an energy band gap of 2.37 eV, suggesting potential applications in optoelectronic devices. BET surface area analysis revealed a surface area of 80 m²/g, indicative of the high surface area of the composites, which may enhance their reactivity and performance in various applications. These findings contribute to the understanding of MoS
2 nanoparticles and their potential utilization in fields such as solar cell, catalysis, sensing, and energy storage.
KEYWORDS:
MoS2; Nanoparticles; Optical Properties; Structural; Solar Cell
Copy the following to cite this article:
Sangeethavanathi S, Gowthaman P, Vigneswaran S, Sathishkumar M. Exploring the Structural, Optical and Surface Area Properties of Mos2 Nanoparticles. Mat. Sci. Res. India;21(2).
|
Copy the following to cite this URL:
Sangeethavanathi S, Gowthaman P, Vigneswaran S, Sathishkumar M. Exploring the Structural, Optical and Surface Area Properties of Mos2 Nanoparticles. Mat. Sci. Res. India;21(2). Available from: https://bit.ly/3WM9Mdb
|
Introduction
Molybdenum disulfide (MoS2) nanoparticles have emerged as a cornerstone in materials science, owing to their exceptional chemical and thermal properties, robustness, and versatility. As a two-dimensional material, MoS2 has captivated researchers worldwide for its intriguing electronic and optical characteristics, which hold immense promise for a myriad of applications 1-2. One particularly enticing avenue lies in integrating MoS2 into composite materials, with the aim of augmenting light absorption, capitalizing on its inherent capability to capture photons across the visible spectrum 3. The extensive utilization of MoS2 chalcogenides underscores their significance across diverse fields. With a small band gap, expansive surface area, high electrical conductivity, minimal charge transfer resistance, substantial specific capacitance, remarkable electrocatalytic activity, and exceptional chemical resilience, MoS2 chalcogenides have found utility in a wide array of applications 4. From energy storage to catalysis, their multifaceted properties have propelled them to the forefront of materials research, offering solutions to pressing challenges in various technological domains 5.
As the global demand for renewable energy sources continues to rise, the quest for efficient and cost-effective solar cell technologies has become increasingly paramount. Among the materials investigated for their potential in photovoltaic applications, molybdenum disulfide (MoS2) has emerged as a promising candidate, thanks to its unique electronic, optical, and structural properties 6. MoS2 layered structure, coupled with its excellent electrical conductivity and high carrier mobility, presents exciting opportunities for enhancing the performance of solar cells. Synthesis plays a pivotal role in tailoring the properties of MoS2 for solar cell applications 7. Various synthesis techniques have been explored to produce MoS2 nanostructures with controlled morphology, size, and crystallinity. Among these methods, chemical vapor deposition (CVD), hydrothermal synthesis, and solvothermal synthesis have emerged as prominent routes for fabricating MoS2 and nanostructures with precise control over their properties 8.
Hydrothermal synthesis has emerged as a versatile and efficient method for the fabrication of various nanomaterials with tailored properties for diverse applications. Among these materials, molybdenum disulfide (MoS2) stands out as a promising candidate due to its unique structural and electronic properties, rendering it suitable for applications ranging from catalysis and energy storage to optoelectronics and sensing 9. In recent years, hydrothermal synthesis has gained traction as a preferred route for the preparation of MoS2 nanostructures with controlled morphology, size, and crystallinity. This method offers distinct advantages, including mild reaction conditions, scalability, and the ability to tune the properties of the resulting MoS2 materials through parameter optimization 10. In recent years, significant progress has been made in understanding the synthesis–structure–property relationships of MoS2 for solar cell applications. Researchers have focused on optimizing synthesis parameters, such as temperature, precursor concentration, and reaction time, to tailor the optoelectronic properties of MoS2 and enhance their compatibility with solar cell architectures 11.
Molybdenum disulfide (MoS2) exhibits several important optical properties such as Bandgap, Absorption Spectrum, Photoluminescence, Exciting Binding Energy, Optical Transparency, and Nonlinear Optical Properties 12. MoS2 is a semiconductor with a direct bandgap in the range of 1.1 to 1.9 electron volts (eV), depending on its thickness and structure. This bandgap makes it suitable for optoelectronic applications, including solar cells 13. MoS2 absorbs light predominantly in the visible range of the electromagnetic spectrum, with absorption increasing towards shorter wavelengths. This property allows MoS2 to efficiently capture sunlight, making it advantageous for photovoltaic applications. When excited by photons, MoS2 emits light through a process known as photoluminescence 14.
The emitted light typically falls within the visible to near-infrared range, and the intensity and peak wavelength can be influenced by factors such as layer thickness and defect density 15. MoS2 exhibits strong excitonic effects due to its two-dimensional nature and quantum confinement effects. Excitons, which are bound electron-hole pairs, play a crucial role in the material’s optical properties, including absorption and emission spectra 16. MoS2 is transparent to a wide range of wavelengths beyond its bandgap, particularly in the near-infrared region. This transparency can be advantageous for certain optoelectronic applications, such as photodetectors and light-emitting diodes (LEDs) 17. MoS2 displays nonlinear optical behavior, meaning its optical properties change non-proportionally with the intensity of incident light. This property can be harnessed for applications such as nonlinear optics and optical switching 18. Overall, the optical properties of MoS2 make it a versatile material for various optoelectronic devices, including solar cells, photodetectors, LEDs, and optical modulators. Researchers continue to explore and exploit these properties to develop innovative technologies with improved performance and efficiency.
This article examines the structural, optical, and surface area features of MoS2 nanoparticles, focusing on their essential traits that make them widely useful. By conducting a thorough research using modern characterization techniques, our goal is to uncover the inherent properties of MoS2 nanoparticles, shedding light on potential uses in state-of-the-art applications. Through the detailed examination of the complex relationship between the arrangement of atoms, the way light interacts with the material, and the characteristics of its surface, our goal is to enhance the overall knowledge of materials made from MoS2. This will promote the development of new ideas and progress in the fields of materials science and engineering.
Materials and Methods
The sodium molybdate dihydrate (Na2MoO4 2H2O), thiourea (CH4N2S), and hydrazine hydrate solution (N2H4.xH2O) and sulfuric acid (H2SO4) were all purchased from merk india with analytical quality and required no further purification.
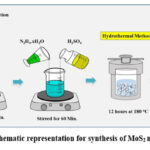
The conventional method for synthesizing MoS2 involves the vigorous mixing of a 1:1 M solution of Na2MoO4.2H2O and CH4N2S in 100 mL of deionized water for duration of 60 minutes. then, a volume of 10 mL of N2H4.xH2O was incrementally introduced into the aforementioned solution, leading to the development of a precipitate with a black hue. The pH of the solution was then modified to 7 with the addition of H2SO4. Subsequently, the black precipitate was transferred to an autoclave and incubated for duration of 12 hours at a temperature of 180°C. The residue was collected, rinsed with deionized water, and subjected to overnight drying at a temperature of 45°C. Furthermore, Figure 1 depicted a schematic illustration of the experimental preparation.
Characterization Details
Structural analysis was performed using a high-resolution X-ray diffractometer (XRD) (XPERT-PRO) to determine the crystalline structure, while Raman spectroscopy (WITech CRM200) provided insights into the molecular vibrational modes. Morphological and elemental composition analysis was carried out using a Field Emission Scanning Electron Microscope (FESEM) (Sigma HV – Carl ZEISS) equipped with Bruker Quantax 200-Z10 Energy-Dispersive X-ray Spectroscopy (EDS) detector. The surface area of the nanoparticles was determined using a Nova 2200e Analyzer through N2 adsorption and desorption processes. Optical properties were investigated using UV-Vis spectrometer (Hitachi-UH5300, λ = 200–900 nm) to assess their absorbance characteristics in the ultraviolet-visible range. These characterization techniques collectively provided a comprehensive understanding of the synthesized MoS2 nanoparticles, paving the way for their potential applications in various technological fields.
Results and Discussion
X-ray diffraction (XRD) analysis was conducted to investigate the crystalline quality and phase purity of MoS2 synthesized via the hydrothermal method and shown in Figure 2 (A). The XRD pattern exhibited distinct peaks at 2θ angles of 14.36º, 32.59º, 39.45º, 49.71º, 58.29º, and 66.51º, corresponding to the (002), (100), (103), (105), (110), and (114) diffraction planes of hexagonal MoS2, respectively. These peaks were in excellent agreement with the standard hexagonal structure of MoS2 (JCPDS no. 37-1492), confirming the successful synthesis of MoS2 via the hydrothermal method. The intensity of the XRD peaks showed that the synthesized MoS2 was crystalline. Sharp and well-defined peaks indicated high crystallinity and phase purity in the synthesized material. This is indicative of the formation of highly crystalline MoS2 nanostructures with well-defined atomic arrangements. Additionally, the XRD analysis provided insights into the size of the crystallites in the synthesized MoS2. The broadening of the XRD peaks observed in the pattern indicated a finite size effect, characteristic of nanomaterials. Using the Scherrer equation 19, the crystallite size of the MoS2 was estimated to be approximately 13 nm. This nanoscale size is consistent with the dimensions expected for materials synthesized via the hydrothermal method and further confirms the formation of MoS2 nanostructures. Overall, the XRD results demonstrate that the hydrothermal method was successful in producing high-quality MoS2 with a well-defined hexagonal structure and nanoscale crystallite size. These findings highlight the potential of hydrothermal synthesis for controlling the crystalline quality and size of MoS2 nanostructures 20.
The Raman spectra revealed (Figure 2B) distinctive peaks corresponding to specific vibrational modes of the MoS2 crystal lattice. The major Raman peaks observed were the E12g and A1g modes, which appeared prominently at 378 cm-1 and 413 cm-1, respectively. These peaks are indicative of the characteristic vibrational modes associated with the hexagonal lattice structure of MoS2 21. The presence of these peaks confirms the successful synthesis of MoS2 with the desired crystalline structure via the hydrothermal method. Additionally, the Raman spectra exhibited the presence of the 2LA (M) peak at 228 cm-1 and 280 cm-1, representing the second-order longitudinal acoustic (2LA) phonon mode. The appearance of this peak further confirms the crystallinity of the synthesized MoS2, as the 2LA (M) mode is a characteristic feature of well-ordered layered structures 15. Furthermore, the Raman spectra revealed the presence of peaks corresponding to the combination of the 2LA (M) and 2TA (M) phonon modes at 456 cm-1. These peaks provide additional evidence of the crystalline quality of the synthesized MoS2, as they arise from the interaction between acoustic and optical phonon modes in the MoS2 crystal lattice.
The FESEM images depicted in Figure 2C clearly showcase the morphology of the MoS2 composites, with individual nanoparticles exhibiting distinct shapes and sizes. The presence of nanoparticles indicates the formation of layered structures characteristic of MoS2, further confirming the successful synthesis of MoS2 via the hydrothermal method. The FESEM images showed flakes-like morphology with varied micrometers, which matched the predicted morphology of MoS2 produced using this technique, demonstrating the hydrothermal method’s ability to regulate material morphology. Additionally, energy-dispersive X-ray spectroscopy (EDX) analysis was performed to confirm the elemental composition of the synthesized MoS2 (Figure 2D). The EDX analysis affirmed the presence of molybdenum (Mo) and sulfur (S) elements, with no detectable impurities observed. This confirms the high purity of the synthesized MoS2 composites and further corroborates the successful synthesis of MoS2 via the hydrothermal method. Overall, the FESEM results demonstrate that the hydrothermal method was effective in producing MoS2 with well-defined flake like morphology, indicative of its microstructured form. The absence of impurities confirmed by EDX analysis further underscores the purity of the synthesized MoS2 composites. These findings highlight the influence of the hydrothermal method on controlling the crystalline morphology and purity of MoS2, making it a promising approach for the synthesis of MoS2 nanostructures for various applications.
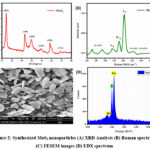 |
Figure 2: Synthesized MoS2 nanoparticles (A) XRD Analysis (B) Raman spectrum (C) FESEM images (D) EDX spectrum
Click here to View Figure
|
The UV-Visible absorption spectra of synthesized MoS2 were analyzed to elucidate their optical properties, shedding light on their potential applications in solar cell technology. Figure 3A illustrates the absorption spectra of MoS2 across the visible spectrum, revealing significant absorption between 400 and 700 nm. The observed absorption peaks in the UV-Visible spectrum indicate the ability of MoS2 to efficiently capture photons within the visible range, a critical requirement for solar cell applications. The absorption of light in this spectral range suggests that MoS2 possess favorable optical properties for harnessing solar energy. To further characterize the optical properties of MoS2, the Tauc equation 22 (Figure 3B) was employed to determine the band gap energies associated with the material. The calculated band gap energy was found to be 2.37 eV, indicating a relatively low band gap for MoS2 synthesized via the hydrothermal method. This low band gap is attributed to the enhanced crystalline structure of the synthesized MoS2, which facilitates efficient light absorption across a broader range of wavelengths. The low band gap energy of MoS2 suggests their potential for effectively collecting larger amounts of light for solar cell applications. By efficiently absorbing photons across the visible spectrum, MoS2 synthesized via the hydrothermal method offer promising opportunities for enhancing the efficiency 23. Overall, the UV-Visible absorption results underscore the favorable optical properties of MoS2 synthesized via the hydrothermal method, positioning them as promising materials for solar cell applications. The low band gap energy, coupled with the significant absorption of light in the visible range, highlights the potential of MoS2 for advancing solar energy conversion technologies 24.
The Berrett-Joyner-Halenda (BJH) adsorption approach was employed to assess the specific surface area, pore volume, and pore size distribution of the synthesized MoS2, providing insights into their surface area properties. Figure 3C illustrates the adsorption-desorption isotherms of nitrogen molecules, which were used to determine the surface area and pore characteristics of the MoS2 nanoparticles. The specific surface area of the MoS2 material was determined to be 80 m²/g, indicating a relatively high surface area conducive to various catalytic applications. The calculated pore size of MoS2 was approximately 18 nm (Figure 3D), suggesting the presence of mesopores within the material. The nitrogen adsorption-desorption isotherm exhibited type IV behavior with hysteresis loops, indicative of mesoporous characteristics in the MoS2 material 25. This type of isotherm suggests the presence of well-defined mesopores, which are desirable for facilitating mass transport and providing active sites for catalytic reactions. Overall, the BET surface analysis results demonstrate that the hydrothermal synthesis method effectively produced MoS2 with a distinct surface area and mesoporous structure 26. The high specific surface area and mesoporous nature of the synthesized MoS2 materials make them promising candidates for various catalytic applications, including energy conversion, environmental remediation, and chemical synthesis. These findings underscore the influence of the hydrothermal method on controlling the surface area properties of MoS2, offering insights into their potential for enhancing catalytic performance in diverse applications. The produced MoS2 nanoparticles exhibit significant light absorption across the visible spectrum, a feature further emphasized by the low band gap energy determined through UV-Visible absorption spectroscopy, highlighting their potential suitability for solar cell applications.
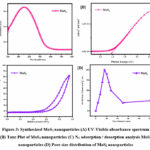 |
Figure 3: Synthesized MoS2 nanoparticles (A) UV-Visible absorbance spectrum (B) Tauc Plot of MoS2 nanoparticles (C) N2 adsorption / desorption analysis MoS2 nanoparticles (D) Pore size distribution of MoS2 nanoparticles
Click here to View Figure
|
The efficacy of the hydrothermal approach in producing MoS2 nanoparticles with favorable structural, optical, and surface area characteristics has been demonstrated. XRD examination confirms the successful creation of MoS2 nanoparticles with the required crystalline structure, as evidenced by the hexagonal phase structure observed. The homogeneity and small size distribution of the produced MoS2 material are indicated by the nanoscale crystallite size, offering advantages in terms of enhancing its optical and catalytic properties. The MoS2 nanoparticles synthesized via the hydrothermal technique demonstrate enhanced light absorption characteristics attributed to their well-defined crystalline structure and nanoscale morphology. Additionally, these MoS2 nanoparticles possess notable specific surface area and mesoporous properties, opening up a wide range of potential applications 27.
Table 1: EDX elements ratio analysis of the prepared MoS2
|
Samples
|
Mo Content %
|
S Content %
|
Total %
|
|
MoS2
|
59.85
|
40.15
|
100
|
MoS2 has numerous optical characteristics that render it very suitable for utilization in solar cell applications. MoS2 possesses a direct bandgap that can be adjusted by manipulating the layer count of the material. By manipulating the crystallite size and morphology of MoS2 layers, it is possible to fine-tune its absorption spectrum to align with the solar spectrum. This, in turn, enhances its efficiency in converting sunlight into electricity 28. MoS2 demonstrates high light absorption in the visible region of the electromagnetic spectrum. This is essential for solar cells, as it allows them to capture a substantial amount of sunlight, resulting in increased efficiency. MoS2 exhibits a high degree of electron mobility, indicating that electrons can readily traverse the material when stimulated by photons 29. This promotes effective charge transfer within the solar cell, minimizing recombination losses and improving the overall performance of the device. MoS2 exhibits high chemical stability under normal environmental circumstances, ensuring the long-term dependability of solar cells that utilize this material. MoS2 possesses a distinctive blend of optical properties, which renders it a highly attractive contender for improving the effectiveness and efficiency of solar cells. This, in turn, has the potential to result in more economically viable and environmentally friendly solar energy harvesting systems 30.
Conclusion
In conclusion, the hydrothermal synthesis method has proven to be highly effective in producing MoS2 nanostructures with desirable characteristics for various applications. Through comprehensive analysis and discussion of results, it is evident that the synthesized MoS2 exhibits exceptional crystalline quality, structural integrity, and optical properties. X-ray diffraction (XRD) analysis confirmed the successful synthesis of MoS2 with a well-defined hexagonal structure and nanoscale crystallite size, indicative of high-quality material. The presence of distinctive peaks corresponding to specific vibrational modes in the Raman spectra further affirmed the crystalline nature of the synthesized MoS2, along with the characteristic flake like morphology observed in FESEM images. Moreover, UV-Visible absorption spectroscopy revealed significant light absorption within the visible spectrum, emphasizing the material’s potential for solar energy applications. The low band gap energy of 2.37 eV suggests efficient photon absorption, further supported by the mesoporous structure observed through BET surface analysis, which enhances catalytic performance. Overall, the findings highlight the versatility and promise of MoS2 synthesized via the hydrothermal method. The material’s combination of superior crystalline quality, optical properties, and surface area characteristics positions it as a viable candidate for a wide range of applications, including solar cells, catalysis, and environmental remediation. The success of this synthesis approach underscores its potential for advancing materials science and contributing to the development of innovative technologies for sustainable energy and environmental solutions.
Acknowledgments
The authors would like to express their sincere gratitude to the Thin Film Research Center, Department of Electronics, Erode Arts and Science College, Erode, for generously providing the necessary research facilities for this study.
Conflict of Interest
The authors declare that there are no conflicts of interest regarding the publication of this manuscript.
Funding Source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Meng Z, Fan J, Chen A, Xie X. Functionalized MoS2 catalysts for CO2 capture and conversion: a review. Mater Today Chem. 2023;29:101449.
CrossRef
- Diao X, Ji N. Rational design of MoS2-based catalysts toward lignin hydrodeoxygenation: Interplay of structure, catalysis, and stability. J Energy Chem. 2023;77:601-631.
CrossRef
- Su HY, Liao W, Sun K. First-principles and microkinetic simulation studies of CO2 hydrogenation mechanism and active site on MoS2 catalyst. Appl Surf Sci. 2023;635:157721.
CrossRef
- Fu W, Ji G, Chen H, Yang S, BaoGuo, Yang H, Huang Z. Molybdenum sulphide modified chelating resin for toxic metal adsorption from acid mine wastewater. Sep Purif Technol. 2020;251:117407.
CrossRef
- Sathishkumar M, Geethalakshmi S. Enhanced photocatalytic and antibacterial activity of Cu:SnO2 nanoparticles synthesized by microwave assisted method. Mater Today Proc. 2020;20(1):54-63.
CrossRef
- Kannan S, Subiramaniyam NP, Sathishkumar M. Investigation on the structural, optical and photocatalytic degradation properties of Zns/Mn:Zns thin films under visible light irradiation. Mater Today Proc. 2021;38(2):907-912.
CrossRef
- Kannan S, Subiramaniyam NP, Sathishkumar M. Effect of annealing temperature and Mn doping on the structural and optical properties of ZnS thin films for enhanced photocatalytic degradation under visible light irradiation. Inorg Chem Commun. 2020;119:108068.
CrossRef
- Sathishkumar M, Saroja M, Venkatachalam M, Gowthaman P, Kannan S, Balamurugan A. rGO encapsulated ZnS photocatalysts for enhanced hydrogen evolution. Mater Lett. 2022;323:132534.
CrossRef
- Mani SK, Saroja M, Venkatachalam M, Rajamanickam T. Antimicrobial activity and photocatalytic degradation properties of zinc sulfide nanoparticles synthesized by using plant extracts. J Nanostruct. 2018;8(2):107-118.
- Kannan S, Subiramaniyam NP, Sathishkumar M. A novel green synthesis approach for improved photocatalytic activity and antibacterial properties of zinc sulfide nanoparticles using plant extract of Acalyphaindica and Tridaxprocumbens. J Mater Sci Mater Electron. 2020;31(12):9846-9859.
CrossRef
- Sathishkumar M, Rajamanickam AT, Saroja M. Characterization, antimicrobial activity and photocatalytic degradation properties of pure and biosynthesized zinc sulfide nanoparticles using plant extracts. J Mater Sci Mater Electron. 2018;29(16):14200-14209.
CrossRef
- Wang, T., Xue, S.-M., Song, T., Ma, L., Liu, Z.-J., & Sun, X.-W. First-principles study of the structural phase transition and optical properties for MoS2 at high pressure. Chemical Physics Letters. 2023; 825:140579.
CrossRef
- Sharma, C., Srivastava, A. K., & Gupta, M. K. Structural, optical and temperature dependent electric modulus property of few-layer MoS2 nanosheets. Physica B: Condensed Matter. 2023; 669: 415290.
CrossRef
- Khan, M. I., Ali, S., Alwadai, N., Haq, I.-u., Irfan, M., Albalawi, H., Almuqrin, A. H., Almoneef, M. M., & Iqbal, M. Structural, electrical and optical properties of heterostructured MoS2/ZnO thin films for potential perovskite solar cells application. Journal of Materials Research and Technology. 2022; 20: 1616-1623.
CrossRef
- Feng, L.-P., Su, J., & Liu, Z.-t. Effect of vacancies on structural, electronic and optical properties of monolayer MoS2: A first-principles study. Journal of Alloys and Compounds. 2014; 613: 122-127.
CrossRef
- Qiu, D., Lee, D. U., Pak, S. W., & Kim, E. K. Structural and optical properties of MoS2 layers grown by successive two-step chemical vapor deposition method. Thin Solid Films. 2015; 587: 47-51.
CrossRef
- Ogata, S., & Imura, K. Optical properties of multilayer MoS2 modified with thermal and shape transformation by laser irradiation. Chemical Physics Letters. 2023; 831: 140859.
CrossRef
- Khan, M., Kumar, S., Mishra, A., Sulania, I., Tripathi, M. N., & Tripathi, A. Study of structural and electronic properties of few-layer MoS2 film. Materials Today: Proceedings. 2022; 57 (Part 1): 100-105.
CrossRef
- Wang L, Zheng X, Yan L, et al. Multiphasic MoS2 activates peroxymonosulfate for efficient removal of oxytetracycline: The dominant role of surface reactive species. Sep Purif Technol. 2023;317:123907.
CrossRef
- Yuan Y, Guo RT, Hong LF, Ji XY, Li ZS, Lin ZD, Pan WG. Recent advances and perspectives of MoS2-based materials for photocatalytic dyes degradation: A review. Colloids Surf A Physicochem Eng Asp. 2021;611:125836.
CrossRef
- Wu MH, Li L, Liu N, et al. Molybdenum disulfide (MoS2) as a co-catalyst for photocatalytic degradation of organic contaminants: A review. Process Saf Environ Prot. 2018;118:40-58.
CrossRef
- Oad N, Chandra P, Mohammad A, Tripathi B, Yoon T. MoS2-based hetero-nanostructures for photocatalytic, photoelectrocatalytic and piezocatalytic remediation of hazardous pharmaceuticals. J Environ Chem Eng. 2023;11(3):109604.
CrossRef
- Chandra P, Mohammad A, Tripathi B, Yoon T. Recent advancements in molybdenum disulfide (MoS2) and its functional nanostructures for photocatalytic and non-photocatalytic organic transformations. Flat Chem. 2022;34:100395.
CrossRef
- Panchal D, Sharma A, Pal S. Engineered MoS2 nanostructures for improved photocatalytic applications in water treatment. Mater Today Sustainability. 2023;21:100264.
CrossRef
- Sethulekshmi AS, Saritha A, Joseph K, Aprem AS, Sisupal SB. MoS2 based nanomaterials: Advanced antibacterial agents for future. J Control Release. 2022;348:158-185.
CrossRef
- Zhang M, Wang K, Zeng S, et al. Visible light-induced antibacterial effect of MoS2: Effect of the synthesis methods. Chem Eng J. 2021;411:128517.
CrossRef
- Xu Q, Zhu P, Zhang J, Liu Y, Cai L, Jiang H, Ji M, Chen J. Electrochemical formation of distinct nanostructured MoS2 with altered antibacterial activity. Mater Lett. 2020;271:127809.
CrossRef
- Patel, A. K., Mishra, R., & Soni, S. K. Performance enhancement of CIGS solar cell with two-dimensional MoS2 hole transport layer. Micro and Nanostructures. 2022; 165: 207195.
CrossRef
- Huang, Y., Shi, X., Liu, X., Cong, R., Sun, Y., Lu, W., & Yu, W. Boosting the photovoltaic performance of MoS2/Si heterojunction solar cells with thiourea-doped MoS2 films. Micro and Nanostructures. 2022; 167: 207241.
CrossRef
- Lu, S., Chen, M., Wang, Y., Li, R., Lin, J., & Zhang, X. Highly efficient MoS2/rGO electrocatalysts for triiodide reduction as Pt-free counter electrode for dye-sensitized solar cells. Solar Energy. 2021; 220:788-795.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.
 , P. Gowthaman1
, P. Gowthaman1 , S. Vigneswaran2
, S. Vigneswaran2 and M. Sathishkumar3
and M. Sathishkumar3


 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal